Estrogen is required for maintaining the quality of cardiac stem cells
- PMID: 33481861
- PMCID: PMC7822545
- DOI: 10.1371/journal.pone.0245166
Estrogen is required for maintaining the quality of cardiac stem cells
Abstract
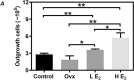
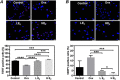
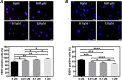
Compared to the age-matched men, the incidence of cardiovascular diseases is lower in premenopausal but higher in postmenopausal women, suggesting the cardio-protective role of estrogen in females. Although cardiac stem cells (CSCs) express estrogen receptors, yet the effects of estrogen on CSCs remain unclear. In this study, we investigated the potential role of estrogen in maintaining the quality of CSCs by in vivo and in vitro experiments. For the in vivo study, estrogen deficiency was induced by ovariectomy in 6-weeks-old C57BL/6 female mice, and then randomly given 17β-estradiol (E2) replacements at a low dose (0.01 mg/60 days) and high dose (0.18 mg/60 days), or vehicle treatment. All mice were killed 2 months after treatments, and heart tissues were collected for ex vivo expansion of CSCs. Compared to age-matched healthy controls, estrogen deficiency slightly decreased the yield of CSCs with significantly lower telomerase activity and more DNA damage. Interestingly, E2 replacements at low and high doses significantly increased the yield of CSCs and reversed the quality impairment of CSCs following estrogen deficiency. For the in vitro study, twice-passaged CSCs from the hearts of adult healthy female mice were cultured with the supplement of 0.01, 0.1, and 1 μM E2 in the medium for 3 days. We found that E2 supplement increased c-kit expression, increased proliferative activity, improved telomerase activity, and reduced DNA damage of CSCs in a dose-dependent manner. Our data suggested the potential role of estrogen in maintaining the quality of CSCs, providing new insight into the cardio-protective effects of estrogen.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

