Text messaging as a tool to improve cancer screening programs (M-TICS Study): A randomized controlled trial protocol
- PMID: 33481914
- PMCID: PMC7822525
- DOI: 10.1371/journal.pone.0245806
Text messaging as a tool to improve cancer screening programs (M-TICS Study): A randomized controlled trial protocol
Update in
-
The use of text messages as an alternative invitation method for breast cancer screening: A randomized controlled trial (M-TICS study).PLoS One. 2024 Aug 29;19(8):e0306720. doi: 10.1371/journal.pone.0306720. eCollection 2024. PLoS One. 2024. PMID: 39208325 Free PMC article. Clinical Trial.
Abstract
Background: Short message service (SMS) based interventions are widely used in healthcare and have shown promising results to improve cancer screening programs. However, more research is still needed to implement SMS in the screening process. We present a study protocol to assess the impact on health and economics of three targeted SMS-based interventions in population-based cancer screening programs.
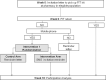
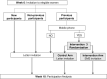
Methods/design: The M-TICs study is a randomized controlled trial with a formal process evaluation. Participants aged 50-69 years identified as eligible from the colorectal cancer (CRC) and breast cancer (BC) screening program of the Catalan Institute of Oncology (Catalonia, Spain) will be randomly assigned to receive standard invitation procedure (control group) or SMS-based intervention to promote participation. Two interventions will be conducted in the CRC screening program: 1) Screening invitation reminder: Those who do not participate in the CRC screening within 6 weeks of invite will receive a reminder (SMS or letter); 2) Reminder to complete and return fecal immunochemical test (FIT) kit: SMS reminder versus no intervention to individuals who have picked up a FIT kit at the pharmacy and they have not returned it after 14 days. The third intervention will be performed in the BC screening program. Women who had been screened previously will receive an SMS invitation or a letter invitation to participate in the screening. As a primary objective we will assess the impact on participation for each intervention. The secondary objectives will be to analyze the cost-effectiveness of the interventions and to assess participants' perceptions.
Expected results: The results from this randomized controlled trial will provide important empirical evidence for the use of mobile phone technology as a tool for improving population-based cancer screening programs. These results may influence the cancer screening invitation procedure in future routine practice.
Trial registration: Registry: NCT04343950 (04/09/2020); clinicaltrials.gov.
Conflict of interest statement
Authors declare funding from ISCIII; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Public Health Pap. Geneva, Switzerland: World Health Organization; 1968.
-
- L 327/34 council recommendation of 2 December 2003 on cancer screening (2003/878/EC). Off. J. Eur. Union, L 327/34-38 2003.
-
- Honein-AbouHaidar GN, Kastner M, Vuong V, Perrier L, Daly C, Rabeneck L, et al. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol. Biomarkers Prev. 2016. p. 907–17. 10.1158/1055-9965.EPI-15-0990 - DOI - PubMed
-
- García M, Borràs JM, Milà N, Espinàs JA, Binefa G, Fernández E, et al. Factors associated with initial participation in a population-based screening for colorectal cancer in Catalonia, Spain: A mixed-methods study. Prev Med (Baltim). 2011;52:265–7. - PubMed
-
- Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition Luxembourg: Office for Official Publications of the European Communities; 2006. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials