Microglia-Secreted Factors Enhance Dopaminergic Differentiation of Tissue- and iPSC-Derived Human Neural Stem Cells
- PMID: 33482100
- PMCID: PMC7878834
- DOI: 10.1016/j.stemcr.2020.12.011
Microglia-Secreted Factors Enhance Dopaminergic Differentiation of Tissue- and iPSC-Derived Human Neural Stem Cells
Abstract
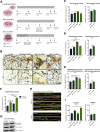
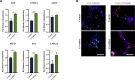
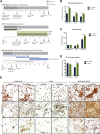
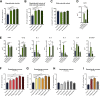
Microglia have recently been established as key regulators of brain development. However, their role in neuronal subtype specification remains largely unknown. Using three different co-culture setups, we show that microglia-secreted factors enhance dopaminergic differentiation of somatic and induced pluripotent stem cell-derived human neural stem cells (NSCs). The effect was consistent across different NSC and microglial cell lines and was independent of prior microglial activation, although restricted to microglia of embryonic origin. We provide evidence that the effect is mediated through reduced cell proliferation and decreased apoptosis and necrosis orchestrated in a sequential manner during the differentiation process. tumor necrosis factor alpha, interleukin-1β, and insulinlike growth factor 1 are identified as key mediators of the effect and shown to directly increase dopaminergic differentiation of human NSCs. These findings demonstrate a positive effect of microglia on dopaminergic neurogenesis and may provide new insights into inductive and protective factors that can stimulate in vitro derivation of dopaminergic neurons.
Keywords: IGF1; IL-1β; NSCs; Parkinson’s disease; TNFα; co-culture; dopamine; microglia; neuron-microglia interaction; secretome analysis.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990;27:229–237. - PubMed
-
- Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A.E., Pluchino S., Martino G., Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell Neurosci. 2006;31:149–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

