Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study
- PMID: 33482113
- PMCID: PMC7816949
- DOI: 10.1016/S2352-3026(20)30429-4
Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study
Erratum in
-
Correction to Lancet Haematol 2021; 8: e185-93.Lancet Haematol. 2021 Jun;8(6):e393. doi: 10.1016/S2352-3026(21)00143-5. Lancet Haematol. 2021. PMID: 34048678 Free PMC article. No abstract available.
Abstract
Background: Haematopoietic stem-cell transplantation (HSCT) recipients are considered at high risk of poor outcomes after COVID-19 on the basis of their immunosuppressed status, but data from large studies in HSCT recipients are lacking. This study describes the characteristics and outcomes of HSCT recipients after developing COVID-19.
Methods: In response to the pandemic, the Center for International Blood and Marrow Transplant Research (CIBMTR) implemented a special form for COVID-19-related data capture on March 27, 2020. All patients-irrespective of age, diagnosis, donor type, graft source, or conditioning regimens-were included in the analysis with data cutoff of Aug 12, 2020. The main outcome was overall survival 30 days after a COVID-19 diagnosis. Overall survival probabilities were calculated using Kaplan-Meier estimator. Factors associated with mortality after COVID-19 diagnosis were examined using Cox proportional hazard models.
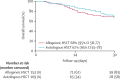
Findings: 318 HSCT recipients diagnosed with COVID-19 were reported to the CIBMTR. The median time from HSCT to COVID-19 diagnosis was 17 months (IQR 8-46) for allogeneic HSCT recipients and 23 months (8-51) for autologous HSCT recipients. The median follow-up of survivors was 21 days (IQR 8-41) for allogeneic HSCT recipients and 25 days (12-35) for autologous HSCT recipients. 34 (18%) of 184 allogeneic HSCT recipients were receiving immunosuppression within 6 months of COVID-19 diagnosis. Disease severity was mild in 155 (49%) of 318 patients, while severe disease requiring mechanical ventilation occurred in 45 (14%) of 318 patients-ie, 28 (15%) of 184 allogeneic HSCT recipients and 17 (13%) of 134 autologous HSCT recipients. At 30 days after the diagnosis of COVID-19, overall survival was 68% (95% CI 58-77) for recipients of allogeneic HSCT and 67% (55-78) for recipients of autologous HSCT. Age 50 years or older (hazard ratio 2·53, 95% CI 1·16-5·52; p=0·020); male sex (3·53; 1·44-8·67; p=0·006), and development of COVID-19 within 12 months of transplantation (2·67, 1·33-5·36; p=0·005) were associated with a higher risk of mortality among allogeneic HSCT recipients, and a disease indication of lymphoma was associated with a higher risk of mortality compared with plasma cell disorder or myeloma (2·41, [1·08-5·38]; p=0·033) in autologous HSCT recipients.
Interpretation: Recipients of autologous and allogeneic HSCT who develop COVID-19 have poor overall survival. These data emphasise the need for stringent surveillance and aggressive treatment measures in HSCT recipients who develop COVID-19.
Funding: American Society of Hematology; Leukemia and Lymphoma Society; National Cancer Institute; National Heart, Lung and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institutes of Health; National Cancer Institute; Health Resources and Services Administration; Office of Naval Research.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Haploidentical hematopoietic cell transplantation is even more advantageous during the COVID-19 pandemic.Pediatr Transplant. 2021 May;25(3):e14004. doi: 10.1111/petr.14004. Epub 2021 Mar 17. Pediatr Transplant. 2021. PMID: 33729657 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
- U01 AI126612/AI/NIAID NIH HHS/United States
- P01 CA023766/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 HL128568/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 HL129472/HL/NHLBI NIH HHS/United States
- P01 CA111412/CA/NCI NIH HHS/United States
- R01 CA215134/CA/NCI NIH HHS/United States
- R01 HL131731/HL/NHLBI NIH HHS/United States
- R01 HL126589/HL/NHLBI NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- R01 AI128775/AI/NIAID NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
- U01 AI069197/AI/NIAID NIH HHS/United States
- R01 CA231141/CA/NCI NIH HHS/United States
- R01 CA218285/CA/NCI NIH HHS/United States
- R01 HL130388/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical