Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study
- PMID: 33482352
- PMCID: PMC7816882
- DOI: 10.1016/j.cmi.2021.01.005
Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study
Abstract
Objectives: To evaluate longitudinally the persistence of humoral immunity for up to 6 months in a cohort of hospital employees with mild coronavirus disease 2019 (COVID-19).
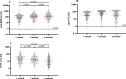
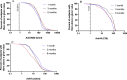
Methods: We measured anti-RBD (receptor binding domain of viral spike protein), anti-N (viral nucleoprotein) and neutralizing antibodies at 1, 3 and 6 months after mostly mild COVID-19 in 200 hospital workers using commercial ELISAs and a surrogate virus neutralization assay.
Results: Antibodies specific for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persisted in all participants for up to 6 months. Anti-RBD geometric mean concentrations (GMCs) progressively increased between months 1 (74.2 U/mL, 95%CI: 62.7-87.8), 3 (103.2 U/mL, 95%CI: 87.9-121.2; p < 0.001), and 6 (123.3 U/mL, 95%CI: 103.4-147.0; p < 0.001) in the whole cohort. Anti-N antibodies were detectable in >97% at all times. Neutralizing antibodies were detectable in 99.5% of participants (195/196) at 6 months post infection. Their GMC progressively decreased between months 1 (20.1 AU/mL, 95%CI: 16.9-24.0), 3 (15.2 AU/mL, 95%CI: 13.2-17.6; p < 0.001) and 6 (9.4 AU/mL, 95%CI: 7.7-11.4; p < 0.001). RBD-ACE2-inhibiting antibody titres and anti-RBD antibody concentrations strongly correlated at each timepoint (all r > 0.86, p < 0.001). Disease severity was associated with higher initial anti-RBD and RBD-ACE2-inhibiting antibody titres, but not with their kinetics.
Conclusions: Neutralizing antibodies persisted at 6 months in almost all participants, indicating more durability than initially feared. Anti-RBD antibodies persisted better and even increased over time, possibly related to the preferential detection of progressively higher-affinity antibodies.
Keywords: Antibody persistence; COVID-19; Humoral immunity; Long-term immunity; Long-term protection; Persistence; SARS-CoV-2.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

