COVID-19 in ocrelizumab-treated people with multiple sclerosis
- PMID: 33482590
- PMCID: PMC7772086
- DOI: 10.1016/j.msard.2020.102725
COVID-19 in ocrelizumab-treated people with multiple sclerosis
Abstract
Background: There are limited data on the impact of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on people with multiple sclerosis (MS).
Objective: To better understand SARS-CoV-2 infection in ocrelizumab-treated people with MS.
Methods: Internal Roche/Genentech data sources: Cases of COVID-19 from ongoing Roche/Genentech clinical trials and from post-marketing use of ocrelizumab until July 31, 2020 were identified and assessed using descriptive statistics. External real-world data (RWD) source: An MS COVID-19 cohort and an ocrelizumab-treated MS COVID-19 cohort were identified and assessed from the OPTUMⓇ de-identified COVID-19 electronic health record (EHR) database.
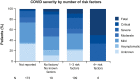
Results: Roche/Genentech clinical trial data: There were 51 (1.3%) suspected or confirmed cases of COVID-19 identified from 4,000 patients ongoing in 10 Roche/Genentech clinical trials. Of these, 26 (51%) were confirmed COVID-19 and 25 (49%) were suspected COVID-19. Sixteen (31.4%) patients were hospitalized. COVID-19 severity was mild to moderate in most patients (35, 68.6%). Ten (19.6%) patients had severe disease and there were three (5.9%) fatal cases. Most patients (43, 84.3%) recovered or were recovering. There was no association apparent between duration of exposure to ocrelizumab and COVID-19. Among COVID-19 patients with previous serum immunoglobulin status (27/51, 52.9%), all (27/27, 100%) had IgG levels within the normal range. Roche/Genentech post-marketing safety database data: There were 307 post-marketing cases of COVID-19 in the Roche/Genentech global safety database. Of these, 263 (85.7%) were confirmed and 44 (14.3%) were suspected COVID-19. 100 (32.6%) patients were hospitalized. COVID-19 was asymptomatic, mild or moderate in 143 (46.6%) patients, severe in 52 (16.9%) patients, and critical in 15 (4.9%) patients. There were 17 (5.5%) fatal cases. Information on severity was not reported in 80 (26.1%) cases. Most patients (211, 68.7%) recovered or were recovering at the time of the report. External RWD data source: As of July 13, 2020, the OPTUMⓇ database included EHRs for almost 1.2 million patients with suspected COVID-19, 130,500 of whom met the criteria for confirmed/clinically diagnosed COVID-19. A total of 357 patients with MS with confirmed COVID-19 were identified. Forty-eight (13.4%) were treated with ocrelizumab, of whom 12 (25.0%) were hospitalized and one died (2.1%). Similar rates of hospitalization, invasive ventilation, and death were observed in the ocrelizumab-treated and non-ocrelizumab-treated MS cohorts. Across the Roche/Genentech and RWD sources assessed, age, male sex, and the presence of comorbidities such as hypertension were associated with a more severe disease course of COVID-19. There was a higher number of comorbidities present in hospitalized versus non-hospitalized patients.
Conclusions: This assessment provides evidence that COVID-19 in ocrelizumab-treated people with MS is predominantly mild to moderate in severity with most patients not requiring hospitalization; in line with data reported from the general population and MS datasets. Risk factors known to be associated with severe COVID-19 outcomes in the general population also appear to influence COVID-19 severity in ocrelizumab-treated people with MS. Case fatality rates for ocrelizumab-treated people with MS were within published ranges for the general population and other MS cohorts.
Keywords: COVID-19; anti-CD20; disease-modifying therapy; multiple sclerosis; ocrelizumab.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Figures




References
-
- Brownlee W, et al. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology; 2020;94:949–952. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

