Valve endothelial-interstitial interactions drive emergent complex calcific lesion formation in vitro
- PMID: 33482604
- PMCID: PMC9437633
- DOI: 10.1016/j.biomaterials.2021.120669
Valve endothelial-interstitial interactions drive emergent complex calcific lesion formation in vitro
Abstract
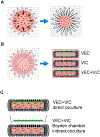
Objective: Calcific aortic valve disease (CAVD) is an actively regulated degenerative disease process. Clinical lesions exhibit marked 3D complexity not represented in current in vitro systems. We here present a unique mechanically stressed 3D culture system that recapitulates valve interstitial cell (VIC) induced matrix calcification through myofibroblastic activation and osteoblastic differentiation. We test the hypothesis that valve endothelial (VEC) - interstitial collaborative interactions modulate the risk and complexity of calcific pathogenesis within mechanically stressed and pro-inflammatory environments.
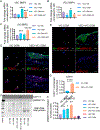
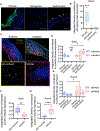
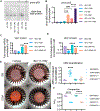
Approach and results: Porcine aortic valve endothelial and interstitial cells (VEC and VIC) were seeded in a mechanically constrained collagen hydrogels alone or in co-culture configurations. Raised 3D VIC-filled lesions formed within 7 days when cultured in osteogenic media (OGM), and surprisingly exacerbated by endothelial coculture. We identified a spatially coordinated pro-endochondral vs. pro-osteogenic signaling program within the lesion. VEC underwent Endothelial-to-Mesenchymal Transformation (EndMT) and populated the lesion center. The spatial complexity of molecular and cellular signatures of this 3D in vitro CAVD system were consistent with human diseased aortic valve histology. SNAI1 was highly expressed in the VEC and subendothelial direct VIC corroborates with human CAVD lesions. Spatial distribution of Sox9 vs. Runx2 expression within the developed lesions (Sox9 peri-lesion vs. Runx2 predominantly within lesions) mirrored their expression in heavily calcified human aortic valves. Finally, we demonstrate the applicability of this platform for screening potential pharmacologic therapies through blocking the canonical NFκB pathway via BAY 11-7082.
Conclusions: Our results establish that VEC actively induce VIC pathological remodeling and calcification via EndMT and paracrine signaling. This mechanically constrained culture platform enables the interrogation of accelerated cell-mediated matrix remodeling behavior underpinned by this cellular feedback circuit. The high fidelity of this complex 3D model system to human CAVD mechanisms supports its use to test mechanisms of intercellular communication in valves and their pharmacological control.
Keywords: Coculture; EndMT; Endochondral ossification; Endothelial dysfunction; Mechanobiology; Osteogenic.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Disclosures
The authors declare no conflicts of interest.
Figures



Similar articles
-
Valvular interstitial cells suppress calcification of valvular endothelial cells.Atherosclerosis. 2015 Sep;242(1):251-260. doi: 10.1016/j.atherosclerosis.2015.07.008. Epub 2015 Jul 17. Atherosclerosis. 2015. PMID: 26232165 Free PMC article.
-
Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation.J Mol Cell Cardiol. 2016 May;94:13-20. doi: 10.1016/j.yjmcc.2016.03.004. Epub 2016 Mar 17. J Mol Cell Cardiol. 2016. PMID: 26996755 Free PMC article.
-
Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling.Am J Pathol. 2013 May;182(5):1922-31. doi: 10.1016/j.ajpath.2013.01.037. Epub 2013 Mar 13. Am J Pathol. 2013. PMID: 23499458 Free PMC article.
-
Oxidative stress and valvular endothelial cells in aortic valve calcification.Biomed Pharmacother. 2023 Jul;163:114775. doi: 10.1016/j.biopha.2023.114775. Epub 2023 Apr 26. Biomed Pharmacother. 2023. PMID: 37116353 Review.
-
Development of calcific aortic valve disease: Do we know enough for new clinical trials?J Mol Cell Cardiol. 2019 Jul;132:189-209. doi: 10.1016/j.yjmcc.2019.05.016. Epub 2019 May 25. J Mol Cell Cardiol. 2019. PMID: 31136747 Review.
Cited by
-
Chondroitin Sulfate Promotes Interstitial Cell Activation and Calcification in an In Vitro Model of the Aortic Valve.Cardiovasc Eng Technol. 2022 Jun;13(3):481-494. doi: 10.1007/s13239-021-00586-z. Epub 2021 Nov 4. Cardiovasc Eng Technol. 2022. PMID: 34735711
-
Inflammation via JAK-STAT/HIF-1α Drives Metabolic Changes in Pentose Phosphate Pathway and Glycolysis That Support Aortic Valve Cell Calcification.Arterioscler Thromb Vasc Biol. 2025 Jul;45(7):e232-e249. doi: 10.1161/ATVBAHA.124.322375. Epub 2025 May 1. Arterioscler Thromb Vasc Biol. 2025. PMID: 40308196
-
Valve endothelial monolayer fissuring via RhoA activity induces 3D calcific aortic valve lesion emergence as revealed by a longitudinal live-imaging platform.bioRxiv [Preprint]. 2025 Jun 26:2025.06.21.660489. doi: 10.1101/2025.06.21.660489. bioRxiv. 2025. PMID: 40666953 Free PMC article. Preprint.
-
Strategies for Development of Synthetic Heart Valve Tissue Engineering Scaffolds.Prog Mater Sci. 2023 Oct;139:101173. doi: 10.1016/j.pmatsci.2023.101173. Epub 2023 Jul 26. Prog Mater Sci. 2023. PMID: 37981978 Free PMC article.
-
Models for calcific aortic valve disease in vivo and in vitro.Cell Regen. 2024 Mar 1;13(1):6. doi: 10.1186/s13619-024-00189-8. Cell Regen. 2024. PMID: 38424219 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

