Sulfadoxine-pyrimethamine parasitological efficacy against Plasmodium falciparum among pregnant women and molecular markers of resistance in Zambia: an observational cohort study
- PMID: 33482823
- PMCID: PMC7821718
- DOI: 10.1186/s12936-021-03596-3
Sulfadoxine-pyrimethamine parasitological efficacy against Plasmodium falciparum among pregnant women and molecular markers of resistance in Zambia: an observational cohort study
Abstract
Background: The World Health Organization recommends the provision of intermittent preventive treatment during pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) at 4-week intervals from gestational week 13 to delivery in areas of moderate to high malaria transmission intensity. However, the effect of IPTp-SP has been compromised in some areas due to parasite resistance, raising the importance of parasitological and chemoprophylactic surveillance, and monitoring SP-resistance markers in the Plasmodium falciparum population.
Methods: Between November 2013 and April 2014 in Nchelenge, Zambia, 1086 pregnant women received IPTp-SP at antenatal-care bookings. Blood samples were collected on day 0, and on day 28 post-treatment to test for malaria parasites and to estimate SP parasitological efficacy in the treatment and prevention of parasitaemia. A random sample of 96, day 0 malaria-positive samples were analysed to estimate the prevalence of SP-resistance markers in the P. falciparum population.
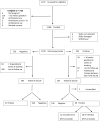
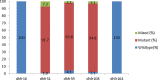
Results: The overall parasitological and prophylactic failure among women who had paired day 0 and day 28 blood slides was 18.6% (95% CI 15.5, 21.8; 109 of 590). Among pregnant women who had asymptomatic parasitaemia on day 0, the day 28 PCR-uncorrected parasitological failure was 30.0% (95% CI 23.7, 36.2; 62 of 207) and the day 28 PCR-corrected parasitological failure was 15.6% (95% CI: 10.6, 20.6; 32 of 205). Among women who tested negative at day 0, 12.3% (95% CI: 9.0, 15.6; 47 of 383) developed parasitaemia at day 28. Among the 96 malaria-positive samples assayed from day 0, 70.8% (95% CI: 60.8, 79.2) contained the DHPS double (Gly-437 + Glu-540) mutation and 92.7% (95% CI: 85.3, 96.5) had the DHFR triple (Asn-108 + Ile-51 + Arg-59) mutation. The quintuple mutation (DHFR triple + DHPS double) and the sextuple mutant (DHFR triple + DHPS double + Arg-581) were found among 68.8% (95% CI: 58.6, 77.3) and 9.4% (95% CI: 4.2, 16.0) of samples, respectively.
Conclusion: The parasitological and chemoprophylactic failure of SP, and the prevalence of resistance markers in Nchelenge is alarmingly high. Alternative therapies are urgently needed to safeguard pregnant women against malarial infection.
Keywords: DHFR triple mutation (asn-108 + ile-51 + arg-59); DHPS double mutation (gly-437 + glu-540); Intermittent preventive treatment of malaria in pregnancy (IPTp); Quintuple mutation (DHFR triple + DHPS double); Sextuple mutation (DHFR triple + DHPS double + arg-581); Sulfadoxine-pyrimethamine (SP).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Efficacy of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy, Mansa, Zambia.Malar J. 2014 Jun 9;13:227. doi: 10.1186/1475-2875-13-227. Malar J. 2014. PMID: 24909578 Free PMC article.
-
High prevalence of dhfr and dhps molecular markers in Plasmodium falciparum in pregnant women of Nchelenge district, Northern Zambia.Malar J. 2015 May 6;14:190. doi: 10.1186/s12936-015-0676-5. Malar J. 2015. PMID: 25943379 Free PMC article.
-
In vivo efficacy of sulphadoxine-pyrimethamine for the treatment of asymptomatic parasitaemia in pregnant women in Machinga District, Malawi.Malar J. 2015 May 13;14:197. doi: 10.1186/s12936-015-0710-7. Malar J. 2015. PMID: 25962439 Free PMC article. Clinical Trial.
-
Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis.Lancet Infect Dis. 2019 May;19(5):546-556. doi: 10.1016/S1473-3099(18)30732-1. Epub 2019 Mar 25. Lancet Infect Dis. 2019. PMID: 30922818
-
Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis.Trop Med Int Health. 2015 Dec;20(12):1621-33. doi: 10.1111/tmi.12595. Epub 2015 Sep 30. Trop Med Int Health. 2015. PMID: 26325263
Cited by
-
The prevalence of molecular markers of resistance to sulfadoxine-pyrimethamine among pregnant women at first antenatal clinic attendance and delivery in the forest-savannah area of Ghana.PLoS One. 2022 Aug 8;17(8):e0271489. doi: 10.1371/journal.pone.0271489. eCollection 2022. PLoS One. 2022. PMID: 35939419 Free PMC article.
-
Geographical Heterogeneity in Antimalarial Resistance Markers Revealed by Genomic Surveillance in Angola, 2023.medRxiv [Preprint]. 2025 Apr 10:2025.04.08.25325242. doi: 10.1101/2025.04.08.25325242. medRxiv. 2025. PMID: 40297444 Free PMC article. Preprint.
-
Effect of Health Education Intervention on Knowledge and Adherence to Intermittent Preventive Treatment of Malaria in Pregnancy Among Women.Healthcare (Basel). 2025 Jan 8;13(2):105. doi: 10.3390/healthcare13020105. Healthcare (Basel). 2025. PMID: 39857134 Free PMC article.
-
Prevalence of molecular markers associated with Plasmodium falciparum resistance to chloroquine and sulphadoxine/pyrimethamine in three malaria endemic local areas of Benue State, Nigeria.Pan Afr Med J. 2025 Mar 13;50:73. doi: 10.11604/pamj.2025.50.73.45826. eCollection 2025. Pan Afr Med J. 2025. PMID: 40519805 Free PMC article.
-
Diagnosis of malaria in pregnancy: accuracy of CareStart™ malaria Pf/PAN against light microscopy among symptomatic pregnant women at the Central Hospital in Yaoundé, Cameroon.Malar J. 2022 Mar 9;21(1):78. doi: 10.1186/s12936-022-04109-6. Malar J. 2022. PMID: 35264170 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

