Harm reduction program and hepatitis C prevalence in people who inject drugs (PWID) in Iran: an updated systematic review and cumulative meta-analysis
- PMID: 33482831
- PMCID: PMC7825164
- DOI: 10.1186/s12954-020-00441-9
Harm reduction program and hepatitis C prevalence in people who inject drugs (PWID) in Iran: an updated systematic review and cumulative meta-analysis
Abstract
Background: Prevalence of hepatitis C virus (HCV) infection among people who inject drugs (PWID) in Iran is high. Since 2005, the Iranian government has implemented a harm reduction program to control HCV. We aimed to describe the prevalence of HCV antibody (Ab) in Iranian PWID before and after the implementation of harm reduction with cumulative meta-analysis.
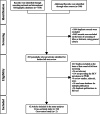
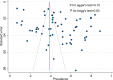
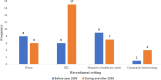
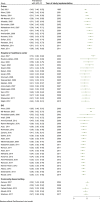
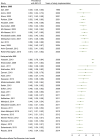
Methods: Following PRISMA guidelines, we conducted a systematic review and meta-analysis of studies published on the seroprevalence of HCV among PWID. We systematically reviewed the literature to identify eligible studies up to December 2018 in international and national databases. Pooled prevalence and 95% confidence intervals were calculated using Der Simonian and Laird method, taking into account conceptual heterogeneity. Subgroup analyses were performed by harm reduction implementation and studies' characteristics to assess the sources of heterogeneity. We used Cochran-Armitage test for the linear trend of the prevalence of HCV Ab among PWID.
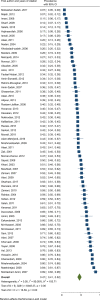
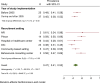
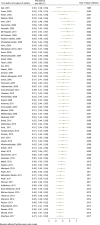
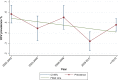
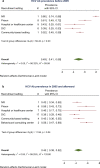
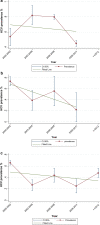
Results: We reviewed 5966 papers and reports and extracted data from 62 eligible records. The pooled HCV Ab prevalence among PWID in Iran was 46.5% (95% confidence interval [95% CI] 41.1-52.0%). Overall, the Cochran-Armitage test for trend indicated a significant decreasing trend of HCV Ab prevalence (P = 0.04). The cumulative meta-analysis showed a slight decline in the prevalence of HCV Ab between the years 2005 and 2018.
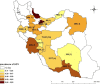
Conclusions: The HCV Ab prevalence among PWID in Iran is high, with a considerable geographical variation. The prevalence of HCV Ab among PWID in Iran slightly decreased after 2005 which could be, at least to some extent, related to the implementation of extensive harm reduction programs in the country.
Keywords: HCV; Harm reduction; Intravenous drug use; Iran.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

