Activation of regulated cell death in the lung of piglets infected with virulent PRRSV-1 Lena strain occurs earlier and mediated by cleaved Caspase-8
- PMID: 33482914
- PMCID: PMC7821682
- DOI: 10.1186/s13567-020-00882-x
Activation of regulated cell death in the lung of piglets infected with virulent PRRSV-1 Lena strain occurs earlier and mediated by cleaved Caspase-8
Abstract
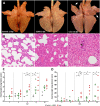
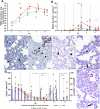
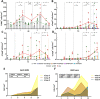
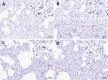
PRRSV-1 virulent strains cause high fever, marked respiratory disease and severe lesions in lung and lymphoid organs. Regulated cell death (RCD), such as apoptosis, necroptosis and pyroptosis, is triggered by the host to interrupt viral replication eliminating infected cells, however, although it seems to play a central role in the immunopathogenesis of PRRSV, there are significant gaps regarding their sequence and activation upon PRRSV-infection. The present study evaluated RCD events by means of caspases expression in the lung of PRRSV-1-infected pigs and their impact on pulmonary macrophage subpopulations and lung lesion. Conventional piglets were intranasally inoculated with the virulent subtype 3 Lena strain or the low virulent subtype 1 3249 strain and euthanised at 1, 3, 6, 8 and 13 dpi. Lena-infected piglets showed severe and early lung damage with a high frequency of PRRSV-N-protein+ cells, depletion of CD163+ cells and high viral load in the lung. The number of TUNEL+ cells was significantly higher than cCasp3+ cells in Lena-infected piglets during the first week post-infection. cCasp8 and to a lesser extent cCasp9 were activated by both PRRSV-1 strains after one week post-infection together with a replenishment of both CD163+ and Arg-1+ pulmonary macrophages. These results highlight the induction of other forms of RCD beyond apoptosis, such as, necroptosis and pyroptosis during the first week post-infection followed by the activation of, mainly, extrinsic apoptosis during the second week post-infection. The recovery of CD163+ macrophages at the end of the study represents an attempt to restore pulmonary macrophage subpopulations lost during the early stages of the infection but also a macrophage polarisation into M2 macrophages.
Keywords: PRRSV-1; cleaved caspase-8; lung; regulated cell death; virulent strain.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Morgan SB, Graham SP, Salguero FJ, Sánchez Cordón PJ, Mokhtar H, Rebel JMJ, Weesendorp E, Bodman-Smith KB, Steinbach F, Frossard JP. Increased pathogenicity of European porcine reproductive and respiratory syndrome virus is associated with enhanced adaptive responses and viral clearance. Vet Microbiol. 2013;163:13–22. doi: 10.1016/j.vetmic.2012.11.024. - DOI - PubMed
-
- Canelli E, Catella A, Borghetti P, Ferrari L, Ogno G, De Angelis E, Corradi A, Passeri B, Bertani V, Sandri G, Bonilauri P, Leung FC, Guazzetti S, Martelli P. Phenotypic characterization of a highly pathogenic Italian porcine reproductive and respiratory syndrome virus (PRRSV) type 1 subtype 1 isolate in experimentally infected pigs. Vet Microbiol. 2017;210:124–133. doi: 10.1016/j.vetmic.2017.09.002. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

