High Remission Rate with Infliximab and Plant-Based Diet as First-Line (IPF) Therapy for Severe Ulcerative Colitis: Single-Group Trial
- PMID: 33482946
- PMCID: PMC7931990
- DOI: 10.7812/TPP/19.166
High Remission Rate with Infliximab and Plant-Based Diet as First-Line (IPF) Therapy for Severe Ulcerative Colitis: Single-Group Trial
Abstract
Introduction: About one-third of patients with severe ulcerative colitis (UC) do not respond to corticosteroid therapy and receive rescue therapy with infliximab or cyclosporine. Up to 20% of such patients fail to respond to rescue therapy and undergo colectomy.
Objective: We investigated the outcomes of infliximab and a plant-based diet (PBD) as first-line therapy for severe UC.
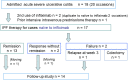
Methods: Patients with severe UC defined by the Truelove and Witts criteria were admitted and given standard induction therapy with infliximab (5.0 mg/kg-7.5 mg/kg) at 0, 2, and 6 weeks. Additionally, they received a PBD. The primary endpoint was remission or colectomy in the induction phase and 1 year after discharge. Secondary endpoints were changes in inflammatory markers in the induction phase and the PBD score at baseline and follow-up. A higher PBD score indicates greater adherence to a PBD.
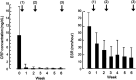
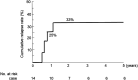
Results: Infliximab and PBD as first-line therapy was administered in 17 cases. The remission rate was 76% (13/17), and the colectomy rate was 6% (1/17) in the induction phase. C-reactive protein values and the erythrocyte sedimentation rate significantly decreased at week 6 from 9.42 mg/dL to 0.33 mg/dL and from 59 to 17 mm/h, respectively (p < 0.0001). At 1-year follow-up, the cumulative relapse rate was 25%, and there were no additional colectomy cases. Mean PBD scores of 27.7 at 1 year and 23.8 at 4 years were significantly higher than baseline scores of 8.3 and 9.9, respectively (p < 0.0001 and p = 0.0391).
Conclusion: This new first-line therapy for severe UC demonstrated a higher remission rate and lower colectomy rate than with the current modality.
Copyright © 2020 The Permanente Press. All rights reserved.
Figures
References
-
- Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of adalimumab on clinical outcomes and health-related quality of life among patients with ulcerative colitis in a clinical setting: results from InspirADA. J Crohn’s Colitis 2017 Oct 27;11(11):1317-25. 10.1093/ecco-jcc/jjx093 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

