Repopulation of decellularised articular cartilage by laser-based matrix engraving
- PMID: 33483297
- PMCID: PMC7910698
- DOI: 10.1016/j.ebiom.2020.103196
Repopulation of decellularised articular cartilage by laser-based matrix engraving
Abstract
Background: In spite of advances in the treatment of cartilage defects using cell and scaffold-based therapeutic strategies, the long-term outcome is still not satisfying since clinical scores decline years after treatment. Scaffold materials currently used in clinical settings have shown limitations in providing suitable biomechanical properties and an authentic and protective environment for regenerative cells. To tackle this problem, we developed a scaffold material based on decellularised human articular cartilage.
Methods: Human articular cartilage matrix was engraved using a CO2 laser and treated for decellularisation and glycosaminoglycan removal. Characterisation of the resulting scaffold was performed via mechanical testing, DNA and GAG quantification and in vitro cultivation with adipose-derived stromal cells (ASC). Cell vitality, adhesion and chondrogenic differentiation were assessed. An ectopic, unloaded mouse model was used for the assessment of the in vivo performance of the scaffold in combination with ASC and human as well as bovine chondrocytes. The novel scaffold was compared to a commercial collagen type I/III scaffold.
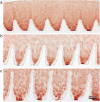
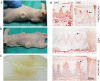
Findings: Crossed line engravings of the matrix allowed for a most regular and ubiquitous distribution of cells and chemical as well as enzymatic matrix treatment was performed to increase cell adhesion. The biomechanical characteristics of this novel scaffold that we term CartiScaff were found to be superior to those of commercially available materials. Neo-tissue was integrated excellently into the scaffold matrix and new collagen fibres were guided by the laser incisions towards a vertical alignment, a typical feature of native cartilage important for nutrition and biomechanics. In an ectopic, unloaded in vivo model, chondrocytes and mesenchymal stromal cells differentiated within the incisions despite the lack of growth factors and load, indicating a strong chondrogenic microenvironment within the scaffold incisions. Cells, most noticeably bone marrow-derived cells, were able to repopulate the empty chondrocyte lacunae inside the scaffold matrix.
Interpretation: Due to the better load-bearing, its chondrogenic effect and the ability to guide matrix-deposition, CartiScaff is a promising biomaterial to accelerate rehabilitation and to improve long term clinical success of cartilage defect treatment.
Funding: Austrian Research Promotion Agency FFG ("CartiScaff" #842455), Lorenz Böhler Fonds (16/13), City of Vienna Competence Team Project Signaltissue (MA23, #18-08).
Keywords: Cartilage regeneration; Decellularisation; Ectopic animal model; Laser engraving; Mechanical testing; Repopulation.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests Dr. Grillari reports that he is Co-founder and shareholder of Evercyte GmbH, the company providing the ASC/TERT1 cells used in this study. Dr. Nürnberger reports grants from Austrian Research Promotion Agency FFG, grants from Lorenz Böhler Society, during the conduct of the study; In addition, Dr. Nürnberger and Dr. Redl have a patent WO 2018220047 A1 issued. Dr. Redl CEO of Trauma Care Consult. Dr. Heimel and Mrs. Schneider report grants from Austrian Research Promotion Agency FFG grants from Lorenz Böhler Society, during the conduct of the study. Dr. Wolbank reports grants from Österreichische Forschungsförderungsgesellschaft, during the conduct of the study.
Figures










References
-
- Aldrian S., Zak L., Wondrasch B., Albrecht C., Stelzeneder B., Binder H., Kovar F., Trattnig S., Marlovits S. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42(11):2680–2688. doi: 10.1177/0363546514548160. - DOI - PubMed
-
- Zak L., Albrecht C., Wondrasch B., Widhalm H., Vekszler G., Trattnig S., Marlovits S., Aldrian S. Results 2 years after matrix-associated autologous chondrocyte transplantation using the novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med. 2014;42(7):1618–1627. doi: 10.1177/0363546514532337. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

