Inactivation of GalU Leads to a Cell Wall-Associated Polysaccharide Defect That Reduces the Susceptibility of Enterococcus faecalis to Bacteriolytic Agents
- PMID: 33483312
- PMCID: PMC8091619
- DOI: 10.1128/AEM.02875-20
Inactivation of GalU Leads to a Cell Wall-Associated Polysaccharide Defect That Reduces the Susceptibility of Enterococcus faecalis to Bacteriolytic Agents
Abstract
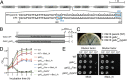
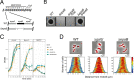
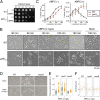
Enterococcal plasmid-encoded bacteriolysin Bac41 is a selective antimicrobial system that is considered to provide a competitive advantage to Enterococcus faecalis cells that carry the Bac41-coding plasmid. The Bac41 effector consists of the secreted proteins BacL1 and BacA, which attack the cell wall of the target E. faecalis cell to induce bacteriolysis. Here, we demonstrated that galU, which encodes UTP-glucose-1-phosphate uridylyltransferase, is involved in susceptibility to the Bac41 system in E. faecalis Spontaneous mutants that developed resistance to the antimicrobial effects of BacL1 and BacA were revealed to carry a truncation deletion of the C-terminal amino acid (aa) region 288 to 298 of the translated GalU protein. This truncation resulted in the depletion of UDP-glucose, leading to a failure to utilize galactose and produce the enterococcal polysaccharide antigen (EPA), which is expressed abundantly on the cell surface of E. faecalis This cell surface composition defect that resulted from galU or EPA-specific genes caused an abnormal cell morphology, with impaired polarity during cell division and alterations of the limited localization of BacL1 Interestingly, these mutants had reduced susceptibility to beta-lactams besides Bac41, despite their increased susceptibility to other bacteriostatic antimicrobial agents and chemical detergents. These data suggest that a complex mechanism of action underlies lytic killing, as exogenous bacteriolysis induced by lytic bacteriocins or beta-lactams requires an intact cell physiology in E. faecalisIMPORTANCE Cell wall-associated polysaccharides of bacteria are involved in various physiological characteristics. Recent studies demonstrated that the cell wall-associated polysaccharide of Enterococcus faecalis is required for susceptibility to bactericidal antibiotic agents. Here, we demonstrated that a galU mutation resulted in resistance to the enterococcal lytic bacteriocin Bac41. The galU homologue is reported to be essential for the biosynthesis of species-specific cell wall-associated polysaccharides in other Firmicutes In E. faecalis, the galU mutant lost the E. faecalis-specific cell wall-associated polysaccharide EPA (enterococcal polysaccharide antigen). The mutant also displayed reduced susceptibility to antibacterial agents and an abnormal cell morphology. We demonstrated here that galU was essential for EPA biosynthesis in E. faecalis, and EPA production might underlie susceptibility to lytic bacteriocin and antibiotic agents by undefined mechanisms.
Keywords: Enterococcus; Enterococcus faecalis; UDP-glucose phosphorylase; antibiotic resistance; bacteriocin; beta-lactam; beta-lactams; cell death; cell morphology; galactose; imaging; morphogenesis; polysaccharide.
Copyright © 2021 American Society for Microbiology.
Figures



References
-
- Nes IF, Clewell DB, Ike Y, Shankar N, Diep DB. 2014. Enterococcal bacteriocins and antimicrobial proteins that contribute to niche control. In Gilmore MS, Clewell DB, Ike Y, et al.. (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

