Color Tuning of Face-Selective Neurons in Macaque Inferior Temporal Cortex
- PMID: 33483324
- PMCID: PMC8174038
- DOI: 10.1523/ENEURO.0395-20.2020
Color Tuning of Face-Selective Neurons in Macaque Inferior Temporal Cortex
Abstract
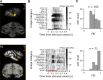
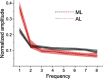
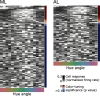
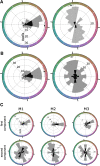
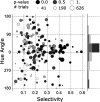
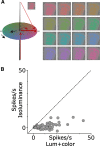
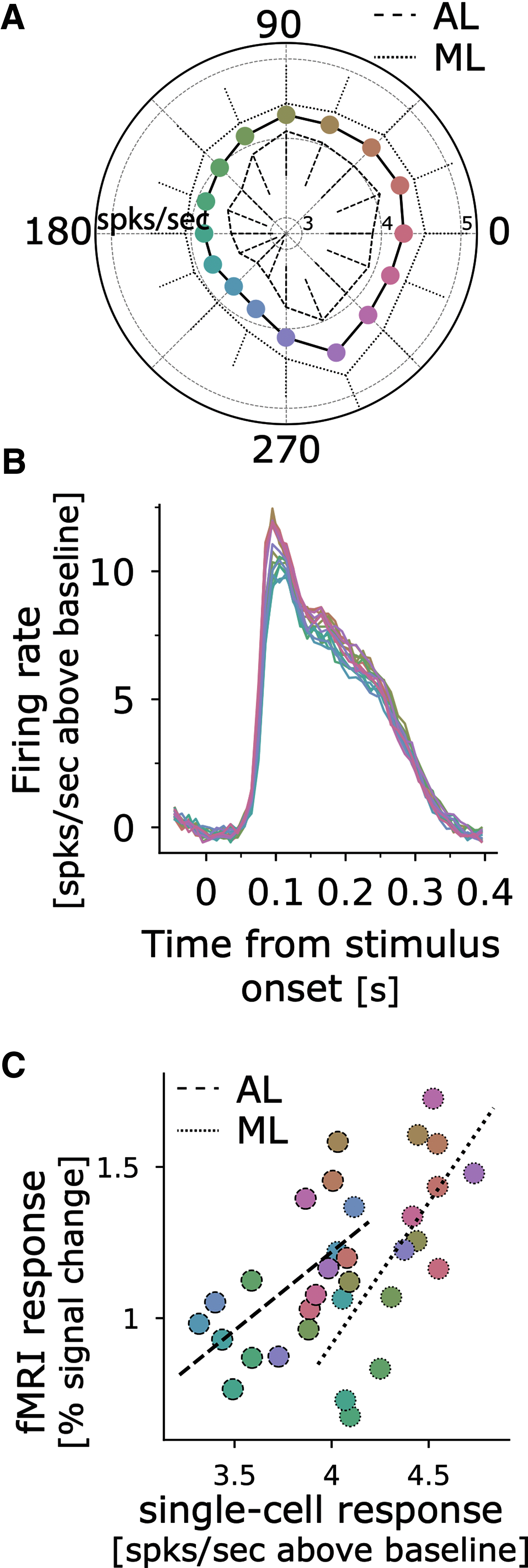
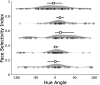
What role does color play in the neural representation of complex shapes? We approached the question by measuring color responses of face-selective neurons, using fMRI-guided microelectrode recording of the middle and anterior face patches of inferior temporal cortex (IT) in rhesus macaques. Face-selective cells responded weakly to pure color (equiluminant) photographs of faces. But many of the cells nonetheless showed a bias for warm colors when assessed using images that preserved the luminance contrast relationships of the original photographs. This bias was also found for non-face-selective neurons. Fourier analysis uncovered two components: the first harmonic, accounting for most of the tuning, was biased toward reddish colors, corresponding to the L>M pole of the L-M cardinal axis. The second harmonic showed a bias for modulation between blue and yellow colors axis, corresponding to the S-cone axis. To test what role face-selective cells play in behavior, we related the information content of the neural population with the distribution of face colors. The analyses show that face-selective cells are not optimally tuned to discriminate face colors, but are consistent with the idea that face-selective cells contribute selectively to processing the green-red contrast of faces. The research supports the hypothesis that color-specific information related to the discrimination of objects, including faces, is handled by neural circuits that are independent of shape-selective cortex, as captured by the multistage parallel processing framework of IT (Lafer-Sousa and Conway, 2013).
Keywords: color vision; face perception; inferior temporal cortex; inferotemporal cortex; neurophysiology; social signaling.
Copyright © 2021 Duyck et al.
Figures




References
-
- Chauhan T, Xiao K, Yates J, Wuerger S (2015) Estimating discrimination ellipsoids for skin images. J Vis 15:820. 10.1167/15.12.820 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
