Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation
- PMID: 33483360
- PMCID: PMC8092751
- DOI: 10.1128/JCM.03149-20
Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation
Abstract
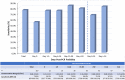
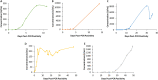
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). Molecular-based testing is used to diagnose COVID-19, and serologic testing of antibodies specific to SARS-CoV-2 is used to detect past infection. While most serologic assays are qualitative, a quantitative serologic assay was recently developed that measures antibodies against the S protein, the target of vaccines. Quantitative antibody determination may help determine antibody titer and facilitate longitudinal monitoring of the antibody response, including antibody response to vaccines. We evaluated the quantitative Roche Elecsys anti-SARS-CoV-2 S assay. Specimens from 167 PCR-positive patients and 103 control specimens were analyzed using the Elecsys anti-SARS-CoV-2 S assay on the cobas e411 (Roche Diagnostics). Analytical evaluation included assessing linearity, imprecision, and analytical sensitivity. Clinical evaluation included assessing clinical sensitivity, specificity, cross-reactivity, positive predictive value (PPV), negative predictive value (NPV), and serial sampling from the same patient. The Elecsys anti-SARS-CoV-2 S assay exhibited its highest sensitivity (84.0%) at 15 to 30 days post-PCR positivity and exhibited no cross-reactivity, a specificity and PPV of 100%, and an NPV between 98.3% and 99.8% at ≥14 days post-PCR positivity, depending on the seroprevalence estimate. Imprecision was <2% at 9.06 U/ml across 6 days, the negative quality control (QC) was consistently negative (<0.40 U/ml), the manufacturer's claimed limit of quantitation of 0.40 U/ml was verified, and linearity across the analytical measuring range was observed, except at the low end (<20 U/ml). Lastly, antibody response showed high interindividual variation in level and time of peak antibody titer and trends over time.
Keywords: COVID-19; SARS-CoV-2; antibody; antibody testing; immunoassays; infectious disease; serology; virology.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Dittrich S, Emperador D, Hooft L, Leeflang MM, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group. 2020. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 6:CD013652. doi: 10.1002/14651858.CD013652. - DOI - PMC - PubMed
-
- Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, Liao P, Qiu J-F, Lin Y, Cai X-F, Wang D-Q, Hu Y, Ren J-H, Tang N, Xu Y-Y, Yu L-H, Mo Z, Gong F, Zhang X-L, Tian W-G, Hu L, Zhang X-X, Xiang J-L, Du H-X, Liu H-W, Lang C-H, Luo X-H, Wu S-B, Cui X-P, Zhou Z, Zhu M-M, Wang J, Xue C-J, Li X-F, Wang L, Li Z-J, Wang K, Niu C-C, Yang Q-J, Tang X-J, Zhang Y, Liu X-M, Li J-J, Zhang D-C, Zhang F, Liu P, Yuan J, Li Q, Hu J-L, Chen J, et al. 2020. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. doi: 10.1038/s41591-020-0897-1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

