MYCN-Amplified Neuroblastoma Is Addicted to Iron and Vulnerable to Inhibition of the System Xc-/Glutathione Axis
- PMID: 33483374
- PMCID: PMC9281612
- DOI: 10.1158/0008-5472.CAN-20-1641
MYCN-Amplified Neuroblastoma Is Addicted to Iron and Vulnerable to Inhibition of the System Xc-/Glutathione Axis
Abstract
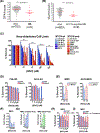
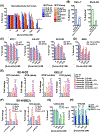
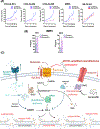
MYCN is amplified in 20% to 25% of neuroblastoma, and MYCN-amplified neuroblastoma contributes to a large percent of pediatric cancer-related deaths. Therapy improvements for this subtype of cancer are a high priority. Here we uncover a MYCN-dependent therapeutic vulnerability in neuroblastoma. Namely, amplified MYCN rewires the cell through expression of key receptors, ultimately enhancing iron influx through increased expression of the iron import transferrin receptor 1. Accumulating iron causes reactive oxygen species (ROS) production, and MYCN-amplified neuroblastomas show enhanced reliance on the system Xc- cystine/glutamate antiporter for ROS detoxification through increased transcription of this receptor. This dependence creates a marked vulnerability to targeting the system Xc-/glutathione (GSH) pathway with ferroptosis inducers. This reliance can be exploited through therapy with FDA-approved rheumatoid arthritis drugs sulfasalazine (SAS) and auranofin: in MYCN-amplified, patient-derived xenograft models, both therapies blocked growth and induced ferroptosis. SAS and auranofin activity was largely mitigated by the ferroptosis inhibitor ferrostatin-1, antioxidants like N-acetyl-L-cysteine, or by the iron scavenger deferoxamine (DFO). DFO reduced auranofin-induced ROS, further linking increased iron capture in MYCN-amplified neuroblastoma to a therapeutic vulnerability to ROS-inducing drugs. These data uncover an oncogene vulnerability to ferroptosis caused by increased iron accumulation and subsequent reliance on the system Xc-/GSH pathway. SIGNIFICANCE: This study shows how MYCN increases intracellular iron levels and subsequent GSH pathway activity and demonstrates the antitumor activity of FDA-approved SAS and auranofin in patient-derived xenograft models of MYCN-amplified neuroblastoma.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 2019;133:144–52 - PubMed
-
- Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta 1999;1413:99–107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

