α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss
- PMID: 33483428
- PMCID: PMC8018774
- DOI: 10.1523/JNEUROSCI.1871-20.2020
α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss
Abstract
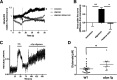
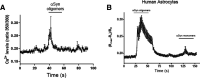
Synaptic and neuronal loss are major neuropathological characteristics of Parkinson's disease. Misfolded protein aggregates in the form of Lewy bodies, comprised mainly of α-synuclein (αSyn), are associated with disease progression, and have also been linked to other neurodegenerative diseases, including Lewy body dementia, Alzheimer's disease, and frontotemporal dementia. However, the effects of αSyn and its mechanism of synaptic damage remain incompletely understood. Here, we show that αSyn oligomers induce Ca2+-dependent release of glutamate from astrocytes obtained from male and female mice, and that mice overexpressing αSyn manifest increased tonic release of glutamate in vivo In turn, this extracellular glutamate activates glutamate receptors, including extrasynaptic NMDARs (eNMDARs), on neurons both in culture and in hippocampal slices of αSyn-overexpressing mice. Additionally, in patch-clamp recording from outside-out patches, we found that oligomerized αSyn can directly activate eNMDARs. In organotypic slices, oligomeric αSyn induces eNMDAR-mediated synaptic loss, which can be reversed by the drug NitroSynapsin. When we expose human induced pluripotent stem cell-derived cerebrocortical neurons to αSyn, we find similar effects. Importantly, the improved NMDAR antagonist NitroSynapsin, which selectively inhibits extrasynaptic over physiological synaptic NMDAR activity, protects synapses from oligomeric αSyn-induced damage in our model systems, thus meriting further study for its therapeutic potential.SIGNIFICANCE STATEMENT Loss of synaptic function and ensuing neuronal loss are associated with disease progression in Parkinson's disease (PD), Lewy body dementia (LBD), and other neurodegenerative diseases. However, the mechanism of synaptic damage remains incompletely understood. α-Synuclein (αSyn) misfolds in PD/LBD, forming Lewy bodies and contributing to disease pathogenesis. Here, we found that misfolded/oligomeric αSyn releases excessive astrocytic glutamate, in turn activating neuronal extrasynaptic NMDA receptors (eNMDARs), thereby contributing to synaptic damage. Additionally, αSyn oligomers directly activate eNMDARs, further contributing to damage. While the FDA-approved drug memantine has been reported to offer some benefit in PD/LBD (Hershey and Coleman-Jackson, 2019), we find that the improved eNMDAR antagonist NitroSynapsin ameliorates αSyn-induced synaptic spine loss, providing potential disease-modifying intervention in PD/LBD.
Keywords: astrocytic glutamate; extrasynaptic NMDARs; synaptic damage; α-synuclein oligomers.
Copyright © 2021 the authors.
Figures






References
-
- Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES, Cox TO, Riddle DM, Zhang B, Trojanowski JQ, Lee VM (2020) Amyloid-β (Aβ) plaques promote seeding and spreading of α-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron 105:260–275. 10.1016/j.neuron.2019.10.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
