Chemoprotective antimalarials identified through quantitative high-throughput screening of Plasmodium blood and liver stage parasites
- PMID: 33483532
- PMCID: PMC7822874
- DOI: 10.1038/s41598-021-81486-z
Chemoprotective antimalarials identified through quantitative high-throughput screening of Plasmodium blood and liver stage parasites
Abstract
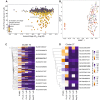
The spread of Plasmodium falciparum parasites resistant to most first-line antimalarials creates an imperative to enrich the drug discovery pipeline, preferably with curative compounds that can also act prophylactically. We report a phenotypic quantitative high-throughput screen (qHTS), based on concentration-response curves, which was designed to identify compounds active against Plasmodium liver and asexual blood stage parasites. Our qHTS screened over 450,000 compounds, tested across a range of 5 to 11 concentrations, for activity against Plasmodium falciparum asexual blood stages. Active compounds were then filtered for unique structures and drug-like properties and subsequently screened in a P. berghei liver stage assay to identify novel dual-active antiplasmodial chemotypes. Hits from thiadiazine and pyrimidine azepine chemotypes were subsequently prioritized for resistance selection studies, yielding distinct mutations in P. falciparum cytochrome b, a validated antimalarial drug target. The thiadiazine chemotype was subjected to an initial medicinal chemistry campaign, yielding a metabolically stable analog with sub-micromolar potency. Our qHTS methodology and resulting dataset provides a large-scale resource to investigate Plasmodium liver and asexual blood stage parasite biology and inform further research to develop novel chemotypes as causal prophylactic antimalarials.
Conflict of interest statement
The authors declare no competing interests.
Figures

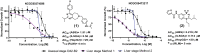

References
-
- WHO . World Malaria Report. Geneva: World Health Organization; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

