Structure-Activity Relationship of Neuroactive Steroids, Midazolam, and Perampanel Toward Mitigating Tetramine-Triggered Activity in Murine Hippocampal Neuronal Networks
- PMID: 33483729
- PMCID: PMC8599726
- DOI: 10.1093/toxsci/kfab007
Structure-Activity Relationship of Neuroactive Steroids, Midazolam, and Perampanel Toward Mitigating Tetramine-Triggered Activity in Murine Hippocampal Neuronal Networks
Abstract
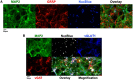
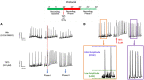
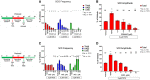
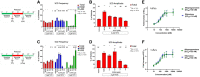
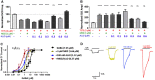
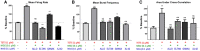
Tetramethylenedisulfotetramine (tetramine or TETS), a potent convulsant, triggers abnormal electrical spike activity (ESA) and synchronous Ca2+ oscillation (SCO) patterns in cultured neuronal networks by blocking gamma-aminobutyric acid (GABAA) receptors. Murine hippocampal neuronal/glial cocultures develop extensive dendritic connectivity between glutamatergic and GABAergic inputs and display two distinct SCO patterns when imaged with the Ca2+ indicator Fluo-4: Low amplitude SCO events (LASE) and High amplitude SCO events (HASE) that are dependent on TTX-sensitive network electrical spike activity (ESA). Acute TETS (3.0 µM) increased overall network SCO amplitude and decreased SCO frequency by stabilizing HASE and suppressing LASE while increasing ESA. In multielectrode arrays, TETS also increased burst frequency and synchronicity. In the presence of TETS (3.0 µM), the clinically used anticonvulsive perampanel (0.1-3.0 µM), a noncompetitive AMPAR antagonist, suppressed all SCO activity, whereas the GABAA receptor potentiator midazolam (1.0-30 µM), the current standard of care, reciprocally suppressed HASE and stabilized LASE. The neuroactive steroid (NAS) allopregnanolone (0.1-3.0 µM) normalized TETS-triggered patterns by selectively suppressing HASE and increasing LASE, a pharmacological pattern distinct from its epimeric form eltanolone, ganaxolone, alphaxolone, and XJ-42, which significantly potentiated TETS-triggered HASE in a biphasic manner. Cortisol failed to mitigate TETS-triggered patterns and at >1 µM augmented them. Combinations of allopregnanolone and midazolam were significantly more effective at normalizing TETS-triggered SCO patterns, ESA patterns, and more potently enhanced GABA-activated Cl- current, than either drug alone.
Keywords: acute neurotoxicity; benzodiazepines; calcium signaling; neuroactive steroids; neuronal networks; pesticides; seizure; tetramine.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Bacci A., Verderio C., Pravettoni E., Matteoli M. (1999). Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur. J. Neurosci. 11, 389–397. - PubMed
-
- Barrueto F., Furdyna P. M., Hoffman R. S., Hoffman R. J., Nelson L. S. (2003a). Status epilepticus from an illegally imported Chinese rodenticide: “tetramine”. J. Toxicol. Clin. Toxicol. 41, 991–994. - PubMed
-
- Barrueto F., Nelson L. S., Hoffman R. S. (2003b). Poisoning by an illegally imported Chinese rodenticide containing tetramethylenedisulfotetramine-New York City, 2002. MMWR Morb. Mortal Wkly. Rep. 52, 199–201. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5210a4.htm. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

