Reducing the effects of control materials based on interchangeability of estimates of day-to-day imprecision between commercial control materials and serum samples
- PMID: 33483963
- PMCID: PMC8059733
- DOI: 10.1002/jcla.23710
Reducing the effects of control materials based on interchangeability of estimates of day-to-day imprecision between commercial control materials and serum samples
Abstract
Background: Reduce the effects in the storage-and-thawing process of commercial control materials based on their interchangeability evaluation.
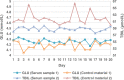
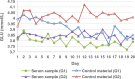
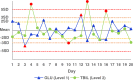
Methods: Seven assays-anti-streptolysin O, complement 3, carcinoembryonic antigen, urea, ferritin, total bilirubin, and glucose-were selected. Commercial control materials and serum samples with similar concentrations were chosen as samples. The experiment was carried out in three stages. In the first stage, the assays with statistical differences in imprecision were screened. In the second stage, two specimens were sealed with parafilm and frozen at -80°C and thawed in the water bath, and the imprecision differences were compared again. Finally, the effective means to reduce the effects were included in the standard operating procedure to repeat confirmation.
Results: In the first stage, there was only a statistical difference (p < 0.05) in the imprecision of glucose and total bilirubin between two specimens, and the imprecision of control materials was higher than the serum samples. In the second stage, glucose imprecision was not statistically different (p > 0.05) and lower than in the first stage. In the third stage, the methods from the second stage were confirmed to be effective at reducing control material effects.
Conclusion: Finding variation factors and confirming and standardizing the measures will help lessen commercial control material effects.
Keywords: control material; imprecision; interchangeability; serum sample.
© 2021 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Hohnadel DC, Sunderman FW, Terhune P, Reid FH, Pomper IH. Comparisons of the precision of replicate analyses of frozen and lyophilized quality control serums. Ann Clin Lab Sci. 1973;3:335‐340. https://pubmed.ncbi.nlm.nih.gov/4756763/ - PubMed
-
- Fuentes‐Arderiu X, Gonzalez‐de‐la‐Presa B. Interchangeability of estimates of day‐to‐day imprecision between commercial control materials and serum pools. Clin Chem. 2002;48:573‐574. https://pubmed.ncbi.nlm.nih.gov/11861454/ - PubMed
-
- Sun H, Shao Y, Liu S, Hu B, Chen B. Evaluation of the effects of calibration modes and sample reconstitution on the analytic precision of 26 clinical chemistry analytes using system measurement procedure. Chin J Lab Med. 2018;41:149‐154. 10.3760/cma.j.issn.1009-9158.2018.02.014 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

