Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19
- PMID: 33484168
- PMCID: PMC8014109
- DOI: 10.1111/all.14746
Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19
Abstract
Background: Many arguments suggest that neutrophils could play a prominent role in COVID-19. However, the role of key components of neutrophil innate immunity in severe forms of COVID-19 has deserved insufficient attention. We aimed to evaluate the involvement of neutrophil elastase, histone-DNA, and DNases in systemic and multi-organ manifestations of COVID-19.
Methods: We performed a multicenter study of markers of neutrophil innate immunity in 155 cases consecutively recruited in a screening center, local hospitals, and two regional university hospitals. The cases were evaluated according to clinical and biological markers of severity and multi-organ manifestations and compared to 35 healthy controls.
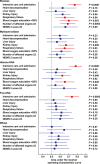
Results: Blood neutrophil elastase, histone-DNA, myeloperoxidase-DNA, and free dsDNA were dramatically increased, and DNase activity was decreased by 10-fold, compared with controls. Neutrophil elastase and histone-DNA were associated with intensive care admission, body temperature, lung damage, and markers of cardiovascular outcomes, renal failure, and increased interleukin-6 (IL-6), IL-8, and CXCR2. Neutrophil elastase was an independent predictor of the computed tomography score of COVID-19 lung damage and the number of affected organs, in multivariate analyses. The increased blood concentrations of NE and neutrophil extracellular traps were related to exacerbation of neutrophil stimulation through IL-8 and CXCR2 increased concentrations and increased serum DAMPs, and to impaired degradation of NETs as a consequence of the dramatic decrease in blood DNase activity.
Conclusion: Our results point out the key role of neutrophil innate immunity exacerbation in COVID-19. Neutrophil elastase and DNase could be potential biomarkers and therapeutic targets of severe systemic manifestations of COVID-19.
Keywords: COVID-19; DNase; innate immunity; myeloperoxidase; neutrophil extracellular traps (NETs).
© 2021 European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
None of the authors had any financial relationships for themselves and their immediate family/significant others.
Figures




References
-
- Mo P, Xing Y, Xiao YU, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020:ciaa270. [Epub ahead of print]. 10.1093/cid/ciaa270 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

