Amelioration of the abnormal phenotype of a new L1 syndrome mouse mutation with L1 mimetics
- PMID: 33484186
- PMCID: PMC12315507
- DOI: 10.1096/fj.202002163R
Amelioration of the abnormal phenotype of a new L1 syndrome mouse mutation with L1 mimetics
Abstract
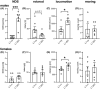
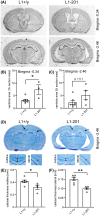
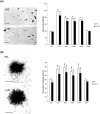
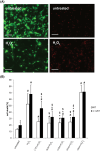
L1 syndrome is a rare developmental disorder characterized by hydrocephalus of varying severity, intellectual deficits, spasticity of the legs, and adducted thumbs. Therapy is limited to symptomatic relief. Numerous gene mutations in the L1 cell adhesion molecule (L1CAM, hereafter abbreviated L1) were identified in L1 syndrome patients, and those affecting the extracellular domain of this transmembrane type 1 glycoprotein show the most severe phenotypes. Previously analyzed rodent models of the L1 syndrome focused on L1-deficient animals or mouse mutants with abrogated cell surface expression of L1, making it difficult to test L1 function-triggering mimetic compounds with potential therapeutic value. To overcome this impasse, we generated a novel L1 syndrome mouse with a mutation of aspartic acid at position 201 in the extracellular part of L1 (p.D201N, hereafter termed L1-201) that displays a cell surface-exposed L1 accessible to the L1 mimetics. Behavioral assessment revealed an increased neurological deficit score and increased locomotor activity in male L1-201 mice carrying the mutation on the X-chromosome. Histological analyses of L1-201 mice showed features of the L1 syndrome, including enlarged ventricles and reduced size of the corpus callosum. Expression levels of L1-201 protein as well as extent of cell surface biotinylation and immunofluorescence labelling of cultured cerebellar neurons were normal. Importantly, treatment of these cultures with the L1 mimetic compounds duloxetine, crotamiton, and trimebutine rescued impaired cell migration and survival as well as neuritogenesis. Altogether, the novel L1 syndrome mouse model provides a first experimental proof-of-principle for the potential therapeutic value of L1 mimetic compounds.
Keywords: L1 syndrome; cell adhesion molecule L1; neuritogenesis; neuronal cell migration; neuronal survival.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
None declared.
Figures


References
-
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19‐26. - PubMed
-
- Sytnyk V, Leshchyns'ka I, Schachner M. Neural cell adhesion molecules of the immunoglobulin superfamily regulate synapse formation, maintenance, and function. Trends Neurosci. 2017;40:295‐308. - PubMed
-
- Schäfer MK, Frotscher M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012;349:39‐48. - PubMed
-
- Harper JR, Prince JT, Healy PA, Stuart JK, Nauman SJ, Stallcup WB. Isolation and sequence of partial cDNA clones of human L1: homology of human and rodent L1 in the cytoplasmic region. J Neurochem. 1991;56:797‐804. - PubMed
-
- Sadoul K, Sadoul R, Faissner A, Schachner M. Biochemical characterization of different molecular forms of the neural cell adhesion molecule L1. J Neurochem. 1988;50:510‐521. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

