Feasibility of intraprocedural integration of cardiac CT to guide left ventricular lead implantation for CRT upgrades
- PMID: 33484216
- PMCID: PMC8647921
- DOI: 10.1111/jce.14896
Feasibility of intraprocedural integration of cardiac CT to guide left ventricular lead implantation for CRT upgrades
Abstract
Background: Optimal positioning of the left ventricular (LV) lead is an important determinant of cardiac resynchronization therapy (CRT) response.
Objective: Evaluate the feasibility of intraprocedural integration of cardiac computed tomography (CT) to guide LV lead implantation for CRT upgrades.
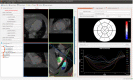
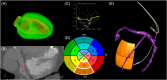
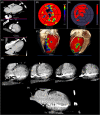
Methods: Patients undergoing LV lead upgrade underwent ECG-gated cardiac CT dyssynchrony and LV scar assessment. Target American Heart Association segment selection was determined using latest non-scarred mechanically activating segments overlaid onto real-time fluoroscopy with image co-registration to guide optimal LV lead implantation. Hemodynamic validation was performed using a pressure wire in the LV cavity (dP/dtmax) ).
Results: 18 patients (male 94%, 55.6% ischemic cardiomyopathy) with RV pacing burden 60.0 ± 43.7% and mean QRS duration 154 ± 30 ms underwent cardiac CT. 10/10 ischemic patients had CT evidence of scar and these segments were excluded as targets. Seventeen out of 18 (94%) patients underwent successful LV lead implantation with delivery to the CT target segment in 15 out of 18 (83%) of patients. Acute hemodynamic response (dP/dtmax ≥ 10%) was superior with LV stimulation in CT target versus nontarget segments (83.3% vs. 25.0%; p = .012). Reverse remodeling at 6 months (LV end-systolic volume improvement ≥15%) occurred in 60% of subjects (4/8 [50.0%] ischemic cardiomyopathy vs. 5/7 [71.4%] nonischemic cardiomyopathy, p = .608).
Conclusion: Intraprocedural integration of cardiac CT to guide optimal LV lead placement is feasible with superior hemodynamics when pacing in CT target segments and favorable volumetric response rates, despite a high proportion of patients with ischemic cardiomyopathy. Multicentre, randomized controlled studies are needed to evaluate whether intraprocedural integration of cardiac CT is superior to standard care.
Keywords: CRT; cardiac CT; image guidance; improving CRT response.
© 2021 The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Figures


References
-
- Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;17368:1585‐1593. - PubMed
-
- Ypenburg C, van Bommel RJ, Delgado V, et al. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol Elsevier. 2008;52:1402‐1409. - PubMed
-
- Behar JM, Mountney P, Toth D, et al. Real‐time X‐MRI‐guided left ventricular lead implantation for targeted delivery of cardiac resynchronization therapy. JACC Clin Electrophysiol Elsevier. 2017;3:803‐814. - PubMed
-
- Daubert JC, Saxon L, Adamson PB, et al. EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow‐up recommendations and management. Heart Rhythm. 2012;2012(9):1524‐1576. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

