An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19
- PMID: 33484240
- PMCID: PMC7939946
- DOI: 10.2196/26292
An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19
Abstract
Background: Chronic low back pain is the most prevalent chronic pain condition worldwide and access to behavioral pain treatment is limited. Virtual reality (VR) is an immersive technology that may provide effective behavioral therapeutics for chronic pain.
Objective: We aimed to conduct a double-blind, parallel-arm, single-cohort, remote, randomized placebo-controlled trial for a self-administered behavioral skills-based VR program in community-based individuals with self-reported chronic low back pain during the COVID-19 pandemic.
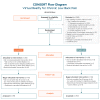
Methods: A national online convenience sample of individuals with self-reported nonmalignant low back pain with duration of 6 months or more and with average pain intensity of 4 or more/10 was enrolled and randomized 1:1 to 1 of 2 daily (56-day) VR programs: (1) EaseVRx (immersive pain relief skills VR program); or (2) Sham VR (2D nature content delivered in a VR headset). Objective device use data and self-reported data were collected. The primary outcomes were the between-group effect of EaseVRx versus Sham VR across time points, and the between-within interaction effect representing the change in average pain intensity and pain-related interference with activity, stress, mood, and sleep over time (baseline to end-of-treatment at day 56). Secondary outcomes were global impression of change and change in physical function, sleep disturbance, pain self-efficacy, pain catastrophizing, pain acceptance, pain medication use, and user satisfaction. Analytic methods included intention-to-treat and a mixed-model framework.
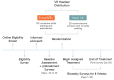
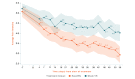
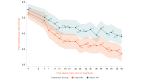
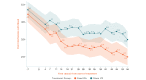
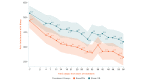
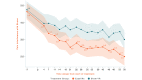
Results: The study sample was 179 adults (female: 76.5%, 137/179; Caucasian: 90.5%, 162/179; at least some college education: 91.1%, 163/179; mean age: 51.5 years [SD 13.1]; average pain intensity: 5/10 [SD 1.2]; back pain duration ≥5 years: 67%, 120/179). No group differences were found for any baseline variable or treatment engagement. User satisfaction ratings were higher for EaseVRx versus Sham VR (P<.001). For the between-groups factor, EaseVRx was superior to Sham VR for all primary outcomes (highest P value=.009), and between-groups Cohen d effect sizes ranged from 0.40 to 0.49, indicating superiority was moderately clinically meaningful. For EaseVRx, large pre-post effect sizes ranged from 1.17 to 1.3 and met moderate to substantial clinical importance for reduced pain intensity and pain-related interference with activity, mood, and stress. Between-group comparisons for Physical Function and Sleep Disturbance showed superiority for the EaseVRx group versus the Sham VR group (P=.022 and .013, respectively). Pain catastrophizing, pain self-efficacy, pain acceptance, prescription opioid use (morphine milligram equivalent) did not reach statistical significance for either group. Use of over-the-counter analgesic use was reduced for EaseVRx (P<.01) but not for Sham VR.
Conclusions: EaseVRx had high user satisfaction and superior and clinically meaningful symptom reduction for average pain intensity and pain-related interference with activity, mood, and stress compared to sham VR. Additional research is needed to determine durability of treatment effects and to characterize mechanisms of treatment effects. Home-based VR may expand access to effective and on-demand nonpharmacologic treatment for chronic low back pain.
Trial registration: ClinicalTrials.gov NCT04415177; https://clinicaltrials.gov/ct2/show/NCT04415177.
International registered report identifier (irrid): RR2-10.2196/25291.
Keywords: COVID-19; behavioral health; chronic pain; low back pain; opioids; pain treatment, randomized controlled trial; virtual reality.
©Laura M Garcia, Brandon J Birckhead, Parthasarathy Krishnamurthy, Josh Sackman, Ian G Mackey, Robert G Louis, Vafi Salmasi, Todd Maddox, Beth D Darnall. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 22.02.2021.
Conflict of interest statement
Conflicts of Interest: LG, TM, and IM are employees of AppliedVR, Inc. JS is president of AppliedVR, Inc. BD is chief science advisor for AppliedVR, Inc. BB, PK, and VS are consultants for AppliedVR, Inc. RL is a prior advisor and minor shareholder of AppliedVR, Inc.
Figures

References
-
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press; 2011. - PubMed
-
- Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009 Feb 09;169(3):251–8. doi: 10.1001/archinternmed.2008.543. http://europepmc.org/abstract/MED/19204216 - DOI - PMC - PubMed
-
- Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, Ferreira PH, Fritz JM, Koes BW, Peul W, Turner JA, Maher CG, Lancet Low Back Pain Series Working Group Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018 Jun 09;391(10137):2368–2383. doi: 10.1016/S0140-6736(18)30489-6. - DOI - PubMed
-
- Williams A, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012 Nov 14;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. http://europepmc.org/abstract/MED/23152245 - DOI - PMC - PubMed
-
- Medicaid Strategies for Non-Opioid Pharmacologic and NonPharmacologic Chronic Pain Management. 2019. [2021-02-03]. https://www.medicaid.gov/sites/default/files/federal-policy-guidance/dow....
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

