Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab Versus Conventional Therapy in Children with X-Linked Hypophosphatemia
- PMID: 33484279
- PMCID: PMC8064984
- DOI: 10.1007/s00223-020-00797-x
Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab Versus Conventional Therapy in Children with X-Linked Hypophosphatemia
Abstract
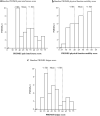
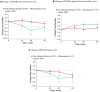
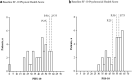
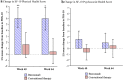
Changing to burosumab, a monoclonal antibody targeting fibroblast growth factor 23, significantly improved phosphorus homeostasis, rickets, lower-extremity deformities, mobility, and growth versus continuing oral phosphate and active vitamin D (conventional therapy) in a randomized, open-label, phase 3 trial involving children aged 1-12 years with X-linked hypophosphatemia. Patients were randomized (1:1) to subcutaneous burosumab or to continue conventional therapy. We present patient-reported outcomes (PROs) from this trial for children aged ≥ 5 years at screening (n = 35), using a Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaire and SF-10 Health Survey for Children. PROMIS pain interference, physical function mobility, and fatigue scores improved from baseline with burosumab at weeks 40 and 64, but changed little with continued conventional therapy. Pain interference scores differed significantly between groups at week 40 (- 5.02, 95% CI - 9.29 to - 0.75; p = 0.0212) but not at week 64. Between-group differences were not significant at either week for physical function mobility or fatigue. Reductions in PROMIS pain interference and fatigue scores from baseline were clinically meaningful with burosumab at weeks 40 and 64 but not with conventional therapy. SF-10 physical health scores (PHS-10) improved significantly with burosumab at week 40 (least-squares mean [standard error] + 5.98 [1.79]; p = 0.0008) and week 64 (+ 5.93 [1.88]; p = 0.0016) but not with conventional therapy (between-treatment differences were nonsignificant). In conclusion, changing to burosumab improved PRO measures, with statistically significant differences in PROMIS pain interference at week 40 versus continuing with conventional therapy and in PHS-10 at weeks 40 and 64 versus baseline.Trial registration: ClinicalTrials.gov NCT02915705.
Keywords: Burosumab; Patient-reported outcomes; Patient-reported outcomes measurement information system; X-linked hypophosphatemia.
Conflict of interest statement
The following authors served as clinical investigators for one or more studies, including this trial, sponsored by Ultragenyx Pharmaceutical Inc. in partnership with Kyowa Kirin International plc: RP, MPW, FHG, CFM, LMW, ON, AAP, JHS, NN, HIC, PP, ES, WH, KM, HT, GSG, AB, FP, and EAI. AAP, NN, FP, and EAI have also received honoraria for serving as advisory board members or for lectures from Ultragenyx Pharmaceutical Inc. RP has received personal fees from Ultragenyx Pharmaceutical Inc. and Kyowa Kirin International plc, a research grant, consultation fees, honoraria, and travel grants from Kyowa Kirin International plc and Alexion UK, and non-financial support from Kyowa Kirin International plc. MPW has received research grant support, honoraria, and travel from Alexion Pharmaceutical Inc. FHG has received personal fees from Kyowa Kirin International plc and research funding from Amgen and Mereo BioPharma. LMW has served as a consultant to Ultragenyx, with funds to LMW’s institution. NN has received personal fees and non-financial support from Kyowa Kirin International plc and Ultragenyx Pharmaceutical Inc. during the conduct of the study, and personal fees and non-financial support (honoraria, consulting fees, and travel support) from Alexion UK outside the submitted work. PP has received research funding from Ultragenyx Pharmaceutical Inc. and is currently an employee of Ascendis Pharma Inc. WH has received honoraria, consulting fees, and travel support from Ultragenyx Pharmaceutical Inc. and research funding, honoraria, and travel support from Kyowa Kirin. GSG has received consulting fees from Ultragenyx Pharmaceutical Inc. AW and WS are employees of Kyowa Kirin International plc. AN is an employee of Chilli Consultancy and has received consultancy fees from Kyowa Kirin International plc to support the development of this manuscript. AC and AS are employees and stockholders of Ultragenyx Pharmaceutical Inc.
Figures
Similar articles
-
Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial.Lancet. 2019 Jun 15;393(10189):2416-2427. doi: 10.1016/S0140-6736(19)30654-3. Epub 2019 May 16. Lancet. 2019. PMID: 31104833 Free PMC article. Clinical Trial.
-
Sustained Efficacy and Safety of Burosumab, a Monoclonal Antibody to FGF23, in Children With X-Linked Hypophosphatemia.J Clin Endocrinol Metab. 2022 Feb 17;107(3):813-824. doi: 10.1210/clinem/dgab729. J Clin Endocrinol Metab. 2022. PMID: 34636899 Free PMC article. Clinical Trial.
-
Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial.Lancet Diabetes Endocrinol. 2019 Mar;7(3):189-199. doi: 10.1016/S2213-8587(18)30338-3. Epub 2019 Jan 9. Lancet Diabetes Endocrinol. 2019. PMID: 30638856 Clinical Trial.
-
Burosumab for Pediatric X-Linked Hypophosphatemia.Curr Osteoporos Rep. 2021 Jun;19(3):271-277. doi: 10.1007/s11914-021-00669-9. Epub 2021 May 10. Curr Osteoporos Rep. 2021. PMID: 33970403 Free PMC article. Review.
-
Efficacy and safety of burosumab compared with conventional therapy in patients with X-linked hypophosphatemia: A systematic review.Arch Endocrinol Metab. 2024 May 17;68:e230242. doi: 10.20945/2359-4292-2023-0242. Arch Endocrinol Metab. 2024. PMID: 38788147 Free PMC article.
Cited by
-
Diagnostic and New Therapeutic Approaches to Two Challenging Pediatric Metabolic Bone Disorders: Hypophosphatasia and X-linked Hypophosphatemic Rickets.Curr Pediatr Rev. 2024;20(4):395-404. doi: 10.2174/0115733963206838231031102750. Curr Pediatr Rev. 2024. PMID: 37927073 Review.
-
X-Linked Hypophosphatemic Rickets: Multisystemic Disorder in Children Requiring Multidisciplinary Management.Front Endocrinol (Lausanne). 2021 Aug 6;12:688309. doi: 10.3389/fendo.2021.688309. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34421819 Free PMC article. Review.
-
Self-Administration of Burosumab in Children and Adults with X-Linked Hypophosphataemia in Two Open-Label, Single-Arm Clinical Studies.Adv Ther. 2023 Apr;40(4):1530-1545. doi: 10.1007/s12325-022-02412-x. Epub 2023 Jan 31. Adv Ther. 2023. PMID: 36719566 Free PMC article.
-
A Study Protocol for the Management of Children With Juvenile Idiopathic Arthritis Based on ePROs.Front Pediatr. 2022 Jul 6;10:905182. doi: 10.3389/fped.2022.905182. eCollection 2022. Front Pediatr. 2022. PMID: 35874559 Free PMC article.
-
Experience of X-linked hypophosphatemic rickets in the Gulf Cooperation Council countries: case series.Endocrinol Diabetes Metab Case Rep. 2024 Apr 11;2024(2):23-0098. doi: 10.1530/EDM-23-0098. Print 2024 Apr 1. Endocrinol Diabetes Metab Case Rep. 2024. PMID: 38614130 Free PMC article.
References
-
- Whyte MP, Carpenter TO, Gottesman GS, Mao M, Skrinar A, San Martin J, Imel EA. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;3:189–199. doi: 10.1016/S2213-8587(18)30338-3. - DOI - PubMed
-
- Linglart A, Biosse-Duplan M, Briot K, Chaussain C, Esterle L, Guillaume-Czitrom S, Kamenicky P, Nevoux J, Prié D, Rothenbuhler A, Wicart P, Harvengt P. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3:R13–R30. doi: 10.1530/EC-13-0103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

