Using an Ordinal Approach to Compare Outcomes Between Vancomycin Versus Ceftaroline or Daptomycin in MRSA Bloodstream Infection
- PMID: 33484408
- PMCID: PMC7954948
- DOI: 10.1007/s40121-021-00401-1
Using an Ordinal Approach to Compare Outcomes Between Vancomycin Versus Ceftaroline or Daptomycin in MRSA Bloodstream Infection
Abstract
Introduction: Vancomycin remains first-line therapy for methicillin-resistant Staphylococcus aureus (MRSA) blood stream infections (BSI); however, its toxicity and reported clinical failures are well established. Binary efficacy endpoints evaluating alternative anti-MRSA therapies leave clinicians deciphering between segregated clinical and safety outcomes and do not provide a comprehensive patient-centered picture of comparative therapies. This study aimed to apply a novel methodology, desirability of outcomes ranking (DOOR), to compare anti-MRSA therapies.
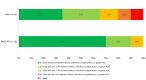
Methods: This was a single-centered, retrospective, cohort of adult patients with MRSA BSI that received vancomycin, daptomycin, or ceftaroline. A previously developed DOOR for S. aureus BSI was adjusted and applied to this cohort to compare vancomycin-treated versus daptomycin/ceftaroline-treated patients. The DOOR had five mutually exclusive ranks: (1) alive without treatment failure, infectious complications, or grade 4 adverse events (AEs); (2) alive with any one of treatment failure, infectious complications, or grade 4 AE; (3) alive with two of treatment failure, infectious complications, or grade 4 AE; (4) alive with all three treatment failure, infectious complications, or grade 4 AE; or (5) deceased.
Results: A total of 43 vancomycin-treated and 13 daptomycin/ceftaroline-treated patients were included. Baseline clinical characteristics were similar, except for higher median serum creatinine in the daptomycin/ceftaroline cohort (0.76 [IQR 0.57, 1.11] vs 1.36 [IQR 1.09, 1.91] mg/dL, P = 0.03). Patients in the daptomycin/ceftaroline cohort had a 92% probability of better outcome using DOOR methodology. Patients treated with daptomycin/ceftaroline experienced less MRSA BSI persistence (0% vs 13.9%), MRSA BSI recurrence (7.8% vs 25.6%), grade 4 AEs (23.1% vs 46.5%), and in-hospital mortality (0% vs 9.3%).
Conclusions: Although limited by sample size, this study demonstrates the potential of DOOR to produce valuable, patient-centered results. Clinicians are encouraged to become familiar with appropriate use and interpretation of DOOR methodology as it will become an increasingly common endpoint in clinical trials.
Keywords: Daptomycin; Desirability of outcomes ranking; S. aureus bacteremia; Vancomycin.
Figures
References
-
- Claeys KC, Zasowski EJ, Casapao AM, et al. Daptomycin improves outcomes regardless of vancomycin MIC in a propensity-matched analysis of methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2016;60(10):5841–5848. doi: 10.1128/AAC.00227-16. - DOI - PMC - PubMed
-
- Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54(1):51–58. doi: 10.1093/cid/cir764. - DOI - PubMed
-
- Murray KP, Zhao JJ, Davis SL, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration > 1 mg/L: a matched cohort study. Clin Infect Dis. 2013;56(11):1562–1569. doi: 10.1093/cid/cit112. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources