Fluorescence Polarization-Based Measurement of Protein-Ligand Interaction in Fungal Cell Lysates
- PMID: 33484500
- PMCID: PMC7850327
- DOI: 10.1002/cpz1.17
Fluorescence Polarization-Based Measurement of Protein-Ligand Interaction in Fungal Cell Lysates
Erratum in
-
Group Correction Statement (Data Availability Statements).Curr Protoc. 2022 Aug;2(8):e552. doi: 10.1002/cpz1.552. Curr Protoc. 2022. PMID: 36005902 Free PMC article. No abstract available.
-
Group Correction Statement (Conflict of Interest Statements).Curr Protoc. 2022 Aug;2(8):e551. doi: 10.1002/cpz1.551. Curr Protoc. 2022. PMID: 36005903 Free PMC article. No abstract available.
Abstract
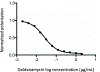
Fungi infect over a billion people worldwide and contribute substantially to human morbidity and mortality despite all available therapies. New antifungal drugs are urgently needed. Decades of study have revealed numerous protein targets of potential therapeutic interest for which potent, fungal-selective ligands remain to be discovered and developed. To measure the binding of diverse small molecule ligands to their larger protein targets, fluorescence polarization (FP) can provide a robust, inexpensive approach. The protocols in this article provide detailed guidance for developing FP-based assays capable of measuring binding affinity in whole cell lysates without the need for purification of the target protein. Applications include screening of libraries to identify novel ligands and the definition of structure-activity relationships to aid development of compounds with improved target affinity and fungal selectivity. © 2021 Wiley Periodicals LLC. Basic Protocol 1: Use of saturation binding curves to optimize tracer and lysate protein concentrations Basic Protocol 2: Establishment of competition binding experiments Support Protocol 1: Preparation of fungal cell lysates Support Protocol 2: Preparation of human HepG2 cell lysate.
Keywords: binding affinity; fluorescence polarization; fungi; whole cell lysate.
© 2021 Wiley Periodicals LLC.
Figures

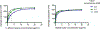

References
-
- Carr PD, Tuckwell D, Hey PM, Simon L, D’Enfert C, Birch M, … Bromley MJ (2010). The transposon impala is activated by low temperatures: Use of a controlled transposition system to identify genes critical for viability of Aspergillus fumigatus. Eukaryotic Cell. 10.1128/EC.00324-09 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

