Enterovirus D68 molecular and cellular biology and pathogenesis
- PMID: 33484714
- PMCID: PMC7949111
- DOI: 10.1016/j.jbc.2021.100317
Enterovirus D68 molecular and cellular biology and pathogenesis
Erratum in
-
Correction: Enterovirus D68 molecular and cellular biology and pathogenesis.J Biol Chem. 2021 Jan-Jun;296:100587. doi: 10.1016/j.jbc.2021.100587. Epub 2021 Apr 9. J Biol Chem. 2021. PMID: 34237885 Free PMC article. No abstract available.
Abstract
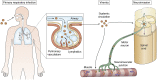
In recent years, enterovirus D68 (EV-D68) has advanced from a rarely detected respiratory virus to a widespread pathogen responsible for increasing rates of severe respiratory illness and acute flaccid myelitis (AFM) in children worldwide. In this review, we discuss the accumulating data on the molecular features of EV-D68 and place these into the context of enterovirus biology in general. We highlight similarities and differences with other enteroviruses and genetic divergence from own historical prototype strains of EV-D68. These include changes in capsid antigens, host cell receptor usage, and viral RNA metabolism collectively leading to increased virulence. Furthermore, we discuss the impact of EV-D68 infection on the biology of its host cells, and how these changes are hypothesized to contribute to motor neuron toxicity in AFM. We highlight areas in need of further research, including the identification of its primary receptor and an understanding of the pathogenic cascade leading to motor neuron injury in AFM. Finally, we discuss the epidemiology of the EV-D68 and potential therapeutic approaches.
Keywords: acute flaccid myelitis; adaptive immunity; enterovirus; innate immunity; virus replication.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Oberste M.S., Maher K., Schnurr D., Flemister M.R., Lovchik J.C., Peters H., Sessions W., Kirk C., Chatterjee N., Fuller S., Hanauer J.M., Pallansch M.A. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J. Gen. Virol. 2004;85:2577–2584. - PubMed
-
- Racaniello V.R. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

