Modulating the foreign body response of implants for diabetes treatment
- PMID: 33484736
- PMCID: PMC8217111
- DOI: 10.1016/j.addr.2021.01.011
Modulating the foreign body response of implants for diabetes treatment
Abstract
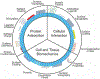
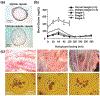
Diabetes Mellitus is a group of diseases characterized by high blood glucose levels due to patients' inability to produce sufficient insulin. Current interventions often require implants that can detect and correct high blood glucose levels with minimal patient intervention. However, these implantable technologies have not reached their full potential in vivo due to the foreign body response and subsequent development of fibrosis. Therefore, for long-term function of implants, modulating the initial immune response is crucial in preventing the activation and progression of the immune cascade. This review discusses the different molecular mechanisms and cellular interactions involved in the activation and progression of foreign body response (FBR) and fibrosis, specifically for implants used in diabetes. We also highlight the various strategies and techniques that have been used for immunomodulation and prevention of fibrosis. We investigate how these general strategies have been applied to implants used for the treatment of diabetes, offering insights on how these devices can be further modified to circumvent FBR and fibrosis.
Keywords: Biomaterials; Diabetes; Encapsulation; Fibrosis; Foreign body response; Immune system; Implants; Sensors; Type I diabetes; Type II diabetes.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures



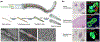

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

