Early changes in synaptic and intrinsic properties of dentate gyrus granule cells in a mouse model of Alzheimer's disease neuropathology and atypical effects of the cholinergic antagonist atropine
- PMID: 33484828
- PMCID: PMC7956160
- DOI: 10.1016/j.nbd.2021.105274
Early changes in synaptic and intrinsic properties of dentate gyrus granule cells in a mouse model of Alzheimer's disease neuropathology and atypical effects of the cholinergic antagonist atropine
Abstract
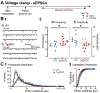
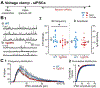
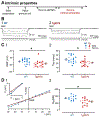
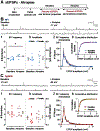
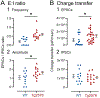
It has been reported that hyperexcitability occurs in a subset of patients with Alzheimer's disease (AD) and hyperexcitability could contribute to the disease. Several studies have suggested that the hippocampal dentate gyrus (DG) may be an important area where hyperexcitability occurs. Therefore, we tested the hypothesis that the principal DG cell type, granule cells (GCs), would exhibit changes at the single-cell level which would be consistent with hyperexcitability and might help explain it. We used the Tg2576 mouse, where it has been shown that hyperexcitability is robust at 2-3 months of age. GCs from 2 to 3-month-old Tg2576 mice were compared to age-matched wild type (WT) mice. Effects of muscarinic cholinergic antagonism were tested because previously we found that Tg2576 mice exhibited hyperexcitability in vivo that was reduced by the muscarinic cholinergic antagonist atropine, counter to the dogma that in AD one needs to boost cholinergic function. The results showed that GCs from Tg2576 mice exhibited increased frequency of spontaneous excitatory postsynaptic potentials/currents (sEPSP/Cs) and reduced frequency of spontaneous inhibitory synaptic events (sIPSCs) relative to WT, increasing the excitation:inhibition (E:I) ratio. There was an inward NMDA receptor-dependent current that we defined here as a novel synaptic current (nsC) in Tg2576 mice because it was very weak in WT mice. Intrinsic properties were distinct in Tg2576 GCs relative to WT. In summary, GCs of the Tg2576 mouse exhibit early electrophysiological alterations that are consistent with increased synaptic excitation, reduced inhibition, and muscarinic cholinergic dysregulation. The data support previous suggestions that the DG contributes to hyperexcitability and there is cholinergic dysfunction early in life in AD mouse models.
Keywords: Acetylcholine; Hyperexcitability; Intrinsic properties; Muscarinic receptors; Synaptic currents; Synaptic potentials.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interest: none.
Figures







Similar articles
-
Increased excitability of dentate gyrus mossy cells occurs early in life in the Tg2576 model of Alzheimer's disease.Alzheimers Res Ther. 2025 May 15;17(1):105. doi: 10.1186/s13195-025-01747-1. Alzheimers Res Ther. 2025. PMID: 40375112 Free PMC article.
-
Increased excitability of dentate gyrus mossy cells occurs early in life in the Tg2576 model of Alzheimer's disease.bioRxiv [Preprint]. 2024 Feb 12:2024.02.09.579729. doi: 10.1101/2024.02.09.579729. bioRxiv. 2024. Update in: Alzheimers Res Ther. 2025 May 15;17(1):105. doi: 10.1186/s13195-025-01747-1. PMID: 38645244 Free PMC article. Updated. Preprint.
-
GABA-activated slow spontaneous inhibitory postsynaptic currents are decreased in dorsal hippocampal dentate gyrus granule cells in an aged mouse model of Alzheimer's disease.J Alzheimers Dis. 2025 Mar;104(2):420-428. doi: 10.1177/13872877251317608. Epub 2025 Feb 16. J Alzheimers Dis. 2025. PMID: 39956943
-
Impaired pattern separation in Tg2576 mice is associated with hyperexcitable dentate gyrus caused by Kv4.1 downregulation.Mol Brain. 2021 Mar 30;14(1):62. doi: 10.1186/s13041-021-00774-x. Mol Brain. 2021. PMID: 33785038 Free PMC article.
-
Aberrant protein S-nitrosylation contributes to hyperexcitability-induced synaptic damage in Alzheimer's disease: Mechanistic insights and potential therapies.Front Neural Circuits. 2023 Feb 2;17:1099467. doi: 10.3389/fncir.2023.1099467. eCollection 2023. Front Neural Circuits. 2023. PMID: 36817649 Free PMC article. Review.
Cited by
-
Effects of altered tau expression on dentate granule cell excitability in mice.Exp Neurol. 2021 Sep;343:113766. doi: 10.1016/j.expneurol.2021.113766. Epub 2021 May 21. Exp Neurol. 2021. PMID: 34029610 Free PMC article.
-
Deleterious and protective effects of epothilone-D alone and in the context of amyloid β- and tau-induced alterations.Front Mol Neurosci. 2023 Oct 12;16:1198299. doi: 10.3389/fnmol.2023.1198299. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37900942 Free PMC article.
-
Dentate Gyrus Granule Cells Show Stability of BDNF Protein Expression in Mossy Fiber Axons with Age, and Resistance to Alzheimer's Disease Neuropathology in a Mouse Model.eNeuro. 2024 Mar 4;11(3):ENEURO.0192-23.2023. doi: 10.1523/ENEURO.0192-23.2023. Print 2024 Mar. eNeuro. 2024. PMID: 38164567 Free PMC article.
-
Neuroprotective Effects of Ferrostatin and Necrostatin Against Entorhinal Amyloidopathy-Induced Electrophysiological Alterations Mediated by voltage-gated Ca2+ Channels in the Dentate Gyrus Granular Cells.Neurochem Res. 2024 Jan;49(1):99-116. doi: 10.1007/s11064-023-04006-7. Epub 2023 Aug 24. Neurochem Res. 2024. PMID: 37615884
-
Urine metabolomics phenotyping and urinary biomarker exploratory in mild cognitive impairment and Alzheimer's disease.Front Aging Neurosci. 2023 Dec 22;15:1273807. doi: 10.3389/fnagi.2023.1273807. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38187356 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

