Nanoparticles for delivery of agents to fetal lungs
- PMID: 33484911
- PMCID: PMC7962939
- DOI: 10.1016/j.actbio.2021.01.024
Nanoparticles for delivery of agents to fetal lungs
Abstract
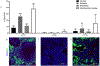
Fetal treatment of congenital lung disease, such as cystic fibrosis, surfactant protein syndromes, and congenital diaphragmatic hernia, has been made possible by improvements in prenatal diagnostic and interventional technology. Delivery of therapeutic agents to fetal lungs in nanoparticles improves cellular uptake. The efficacy and safety of nanoparticle-based fetal lung therapy depends on targeting of necessary cell populations. This study aimed to determine the relative distribution of nanoparticles of a variety of compositions and sizes in the lungs of fetal mice delivered through intravenous and intra-amniotic routes. Intravenous delivery of particles was more effective than intra-amniotic delivery for epithelial, endothelial and hematopoietic cells in the fetal lung. The most effective targeting of lung tissue was with 250nm Poly-Amine-co-Ester (PACE) particles accumulating in 50% and 44% of epithelial and endothelial cells. This study demonstrated that route of delivery and particle composition impacts relative cellular uptake in fetal lung, which will inform future studies in particle-based fetal therapy.
Keywords: Biodegradable nanoparticles; Biodistribution; Fetal therapy; Lung targeting.
Copyright © 2021 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Authors WMS, PMG, ASR, DHS, ASP are inventors of the following: https://patents.justia.com/patent/20200113821
Figures

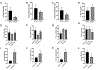
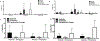
References
-
- Coughlin MA, Werner NL, Gajarski R, Gadepalli S, Hirschl R, Barks J, Treadwell MC, Ladino-Torres M, Kreutzman J, Mychaliska GB, Prenatally diagnosed severe CDH: mortality and morbidity remain high, Journal of pediatric surgery 51(7) (2016) 1091–5. - PubMed
-
- Hamvas A, Cole FS, Nogee LM, Genetic disorders of surfactant proteins, Neonatology 91(4) (2007) 311–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

