Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial
- PMID: 33485468
- PMCID: PMC7825810
- DOI: 10.1016/S1473-3099(20)30942-7
Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial
Erratum in
-
Correction to Lancet Infect Dis 2021; published online Jan 21. https://doi.org/10.1016/S1473-3099(20)30942-7.Lancet Infect Dis. 2021 Apr;21(4):e81. doi: 10.1016/S1473-3099(21)00131-6. Epub 2021 Feb 23. Lancet Infect Dis. 2021. PMID: 33636147 Free PMC article. No abstract available.
-
Correction to Lancet Infect Dis 2021; 21: 637-46.Lancet Infect Dis. 2023 Mar;23(3):e80. doi: 10.1016/S1473-3099(23)00042-7. Epub 2023 Jan 18. Lancet Infect Dis. 2023. PMID: 36681086 Free PMC article. No abstract available.
Abstract
Background: To mitigate the effects of COVID-19, a vaccine is urgently needed. BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine formulated with a toll-like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG) or alum (Algel).
Methods: We did a double-blind, multicentre, randomised, controlled phase 1 trial to assess the safety and immunogenicity of BBV152 at 11 hospitals across India. Healthy adults aged 18-55 years who were deemed healthy by the investigator were eligible. Individuals with positive SARS-CoV-2 nucleic acid and/or serology tests were excluded. Participants were randomly assigned to receive either one of three vaccine formulations (3 μg with Algel-IMDG, 6 μg with Algel-IMDG, or 6 μg with Algel) or an Algel only control vaccine group. Block randomisation was done with a web response platform. Participants and investigators were masked to treatment group allocation. Two intramuscular doses of vaccines were administered on day 0 (the day of randomisation) and day 14. Primary outcomes were solicited local and systemic reactogenicity events at 2 h and 7 days after vaccination and throughout the full study duration, including serious adverse events. Secondary outcome was seroconversion (at least four-fold increase from baseline) based on wild-type virus neutralisation. Cell-mediated responses were evaluated by intracellular staining and ELISpot. The trial is registered at ClinicalTrials.gov (NCT04471519).
Findings: Between July 13 and 30, 2020, 827 participants were screened, of whom 375 were enrolled. Among the enrolled participants, 100 each were randomly assigned to the three vaccine groups, and 75 were randomly assigned to the control group (Algel only). After both doses, solicited local and systemic adverse reactions were reported by 17 (17%; 95% CI 10·5-26·1) participants in the 3 μg with Algel-IMDG group, 21 (21%; 13·8-30·5) in the 6 μg with Algel-IMDG group, 14 (14%; 8·1-22·7) in the 6 μg with Algel group, and ten (10%; 6·9-23·6) in the Algel-only group. The most common solicited adverse events were injection site pain (17 [5%] of 375 participants), headache (13 [3%]), fatigue (11 [3%]), fever (nine [2%]), and nausea or vomiting (seven [2%]). All solicited adverse events were mild (43 [69%] of 62) or moderate (19 [31%]) and were more frequent after the first dose. One serious adverse event of viral pneumonitis was reported in the 6 μg with Algel group, unrelated to the vaccine. Seroconversion rates (%) were 87·9, 91·9, and 82·8 in the 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, and 6 μg with Algel groups, respectively. CD4+ and CD8+ T-cell responses were detected in a subset of 16 participants from both Algel-IMDG groups.
Interpretation: BBV152 led to tolerable safety outcomes and enhanced immune responses. Both Algel-IMDG formulations were selected for phase 2 immunogenicity trials. Further efficacy trials are warranted.
Funding: Bharat Biotech International.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
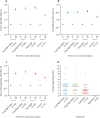

Comment in
-
Optimism and caution for an inactivated COVID-19 vaccine.Lancet Infect Dis. 2021 May;21(5):581-582. doi: 10.1016/S1473-3099(20)30988-9. Epub 2021 Jan 21. Lancet Infect Dis. 2021. PMID: 33485467 Free PMC article. No abstract available.
-
Adjuvantation helps to optimise COVID-19 vaccine candidate.Lancet Infect Dis. 2021 Jul;21(7):891-893. doi: 10.1016/S1473-3099(21)00094-3. Epub 2021 Mar 8. Lancet Infect Dis. 2021. PMID: 33705726 Free PMC article. No abstract available.
References
-
- WHO Draft landscape of COVID-19 candidate vaccines. Dec 8, 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
-
- Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. - PubMed
-
- Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23(suppl):S46–S57. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

