Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration of Depo-Provera
- PMID: 33485608
- PMCID: PMC8051852
- DOI: 10.1016/j.fertnstert.2020.11.002
Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration of Depo-Provera
Abstract
Objective: To evaluate the pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after a single subcutaneous injection in the abdomen of 150 or 300 mg Depo-Provera and compare results to two injections of Depo-SubQ Provera 104 given 3 months apart.
Design: Partially randomized, multicenter, parallel-group study.
Setting: Research unit.
Patient(s): Forty-two women of reproductive age with confirmed ovulatory cycle and body mass index of 18-35 kg/m2.
Intervention(s): Women received a single subcutaneous injection of 150 mg (n = 24) or 300 mg (n = 9) of Depo-Provera or two injections of Depo-SubQ Provera 104 (n = 9).
Main outcome measure(s): Suppression of ovulation as measured by progesterone, serum medroxyprogesterone acetate concentrations, and estimated pharmacokinetics parameters.
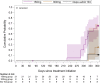
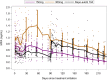
Result(s): No ovulations were observed during 7 months after a single injection of 150 or 300 mg Depo-Provera. The 150 mg group had a similar Cmax as observed over two injection cycles of Depo-SubQ Provera 104 and a similar 6-month trough concentration as the 3-month trough of Depo-SubQ Provera 104.
Conclusion(s): Our pharmacodynamics and pharmacokinetics data provide proof of concept that Depo-Provera (150 mg) may be an effective contraceptive method when injected subcutaneously every 6 months, with up to a 4-week grace period for reinjections.
Clinical trial registration number: NCT02456584.
Ensayo clínico para la evaluación de la farmacocinética y farmacodinámica del acetato de medroxiprogesterona tras la administración subcutánea de Depo-Provera.
Objetivo: Evaluar la farmacocinética y farmacodinámica de acetato de medroxiprogesterona después de una única inyección subcutánea abdominal de 150 o 300 mg de Depo-Provera y comparar los resultados con dos inyecciones de Depo-SubQ Provera 104 administradas con un intervalo de 3 meses.
Diseño: Estudio multicéntrico, parcialmente aleatorizado y en grupos paralelos.
Entorno: Unidad de investigación.
Paciente(s): Cuarenta y dos mujeres en edad reproductiva con ciclos ovulatorios confirmados e índice de masa corporal entre 18 y 35 kg/m2.
Intervención: Las mujeres recibieron una única inyección subcutánea de 150 mg (n=24) o 300 mg (n=9) de Depo-Provera o dos inyecciones de Depo-SubQ Provera 104 (n=9).
Medidas principales de resultado(s): Supresión de la ovulación determinada por progesterona, concentraciones séricas de acetato de medroxyprogesterona y estimación de los parámetros farmacocinéticos.
Resultado(s): No se observaron ovulaciones durante 7 meses después de una única inyección de 150 o 300 mg de Depo-Provera. El grupo de 150 mg tuvo una Cmax similar a la observada con dos ciclos de inyección de Depo-SubQ Provera 104 y una concentración similar durante 6 meses a la de Depo.SubQ Provera 104 durante 3 meses.
Conclusión(es): Nuestros datos farmacodinámicos y farmacocinéticos proporcionan la “prueba de concepto” de que la Depo-Provera (150 mg) puede ser un método contraceptivo eficaz inyectado cada 6 meses vía subcutánea, con hasta 4 semanas de período de gracia para las re-inyecciones.
Palabra(s) clave(s): Medroxiprogesterona acetato depot, subcutánea, contracepción, farmacocinética, supresión de la ovulación.
Keywords: Depot medroxyprogesterone acetate; contraception; pharmacokinetics; subcutaneous; suppression of ovulation.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Using pharmacologic research to efficiently meet acute contraceptive needs.Fertil Steril. 2021 Apr;115(4):903-904. doi: 10.1016/j.fertnstert.2021.02.027. Fertil Steril. 2021. PMID: 33832748 Free PMC article. No abstract available.
References
-
- United Nations, Department of Economics and Social Affairs Contraceptive use by method 2019. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa... Available at:
-
- Lakha F., Henderson C., Glasier A. The acceptability of self-administration of subcutaneous Depo-Provera. Contraception. 2005;72:14–18. - PubMed
-
- Castle W.M., Sapire K.E., Howard K.A. Efficacy and acceptability of injectable medroxyprogesterone. A comparison of 3-monthly and 6-monthly regimens. S Afr Med J. 1978;53:842–845. - PubMed
-
- Ruminjo J.K., Sekadde-Kigondu C.B., Karanja J.G., Rivera R., Nasution M., Nutley T. Comparative acceptability of combined and progestin-only injectable contraceptives in Kenya. Contraception. 2005;72:138–145. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

