Comparison of Two Commercial Platforms and a Laboratory-Developed Test for Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA
- PMID: 33486074
- PMCID: PMC7825913
- DOI: 10.1016/j.jmoldx.2021.01.005
Comparison of Two Commercial Platforms and a Laboratory-Developed Test for Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA
Abstract
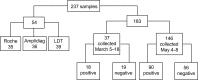
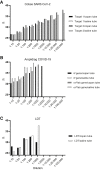
Mitigation of the ongoing coronavirus disease 2019 (COVID-19) pandemic requires reliable and accessible laboratory diagnostic services. In this study, the performance of one laboratory-developed test (LDT) and two commercial tests, cobas SARS-CoV-2 (Roche) and Amplidiag COVID-19 (Mobidiag), were evaluated for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in respiratory specimens. A total of 183 specimens collected from suspected COVID-19 patients were studied with all three methods to compare their performance. In relation to the reference standard, which was established as the result obtained by two of the three studied methods, the positive percent agreement was highest for the cobas test (100%), followed by the Amplidiag test and the LDT (98.9%). The negative percent agreement was lowest for the cobas test (89.4%), followed by the Amplidiag test (98.8%), and the highest value was obtained for the LDT (100%). The dilution series of positive specimens, however, suggests significantly higher sensitivity for the cobas assay in comparison with the other two assays, and the low negative percent agreement value may be due to the same reason. In general, all tested assays performed adequately. Clinical laboratories need to be prepared for uninterrupted high-throughput testing during the coming months to mitigate the pandemic. To ensure no interruption, it is critical that clinical laboratories maintain several simultaneous platforms in their SARS-CoV-2 nucleic acid testing.
Copyright © 2021 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. - PMC - PubMed
-
- Rodino K.G., Espy M.J., Buckwalter S.P., Walchak R.C., Germer J.J., Fernholz E., Boerger A., Schuetz A.N., Yao J.D., Binnicker M.J. Evaluation of saline, phosphate-buffered saline, and minimum essential medium as potential alternatives to viral transport media for SARS-CoV-2 testing. J Clin Microbiol. 2020;58 e00590-20. - PMC - PubMed
-
- Nummi M., Mannonen L., Puolakkainen M. Development of a multiplex real-time PCR assay for detection of Mycoplasma pneumoniae, Chlamydia pneumoniae and mutations associated with macrolide resistance in Mycoplasma pneumoniae from respiratory clinical specimens. Springerplus. 2015;4:684. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

