Biomaterial-guided stem cell organoid engineering for modeling development and diseases
- PMID: 33486104
- PMCID: PMC8629488
- DOI: 10.1016/j.actbio.2021.01.026
Biomaterial-guided stem cell organoid engineering for modeling development and diseases
Abstract
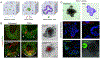
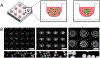
Organoids are miniature models of organs to recapitulate spatiotemporal cellular organization and tissue functionality. The production of organoids has revolutionized the field of developmental biology, providing the possibility to study and guide human development and diseases in a dish. More recently, novel biomaterial-based culture systems demonstrated the feasibility and versatility to engineer and produce the organoids in a consistent and reproducible manner. By engineering proper tissue microenvironment, functional organoids have been able to exhibit spatial-distinct tissue patterning and morphogenesis. This review focuses on enabling technologies in the field of organoid engineering, including the control of biochemical and biophysical cues via hydrogels, as well as size and geometry control via microwell and microfabrication techniques. In addition, this review discusses the enhancement of organoid systems for therapeutic applications using biofabrication and organoid-on-chip platforms, which facilitate the assembly of complex organoid systems for in vitro modeling of development and diseases. STATEMENT OF SIGNIFICANCE: Stem cell organoids have revolutionized the fields of developmental biology and tissue engineering, providing the opportunity to study human organ development and disease progression in vitro. Various works have demonstrated that organoids can be generated using a wide variety of engineering tools, materials, and systems. Specific culture microenvironment is tailored to support the formation, function, and physiology of the organ of interest. This review highlights the importance of cellular microenvironment in organoid culture, the versatility of organoid engineering techniques, and future perspectives to build better organoid systems.
Keywords: Biomaterials; Organoids; Self-organization; Spatial patterning; Stem cell engineering.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

