Cell-penetrating, antioxidant SELENOT mimetic protects dopaminergic neurons and ameliorates motor dysfunction in Parkinson's disease animal models
- PMID: 33486153
- PMCID: PMC7823055
- DOI: 10.1016/j.redox.2020.101839
Cell-penetrating, antioxidant SELENOT mimetic protects dopaminergic neurons and ameliorates motor dysfunction in Parkinson's disease animal models
Abstract
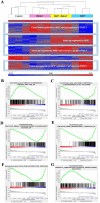
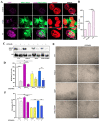
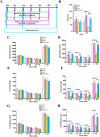
Parkinson's disease (PD) is a neurodegenerative disorder characterized by motor dysfunction for which there is an unmet need for better treatment options. Although oxidative stress is a common feature of neurodegenerative diseases, notably PD, there is currently no efficient therapeutic strategy able to tackle this multi-target pathophysiological process. Based on our previous observations of the potent antioxidant and neuroprotective activity of SELENOT, a vital thioredoxin-like selenoprotein, we designed the small peptide PSELT from its redox active site to evaluate its antioxidant properties in vivo, and its potential polyfunctional activity in PD models. PSELT protects neurotoxin-treated dopaminergic neurons against oxidative stress and cell death, and their fibers against neurotoxic degeneration. PSELT is cell-permeable and acts in multiple subcellular compartments of dopaminergic neurons that are vulnerable to oxidative stress. In rodent models of PD, this protective activity prevented neurodegeneration, restored phosphorylated tyrosine hydroxylase levels, and led to improved motor skills. Transcriptomic analysis revealed that gene regulation by PSELT after MPP+ treatment negatively correlates with that occurring in PD, and positively correlates with that occurring after resveratrol treatment. Mechanistically, a major impact of PSELT is via nuclear stimulation of the transcription factor EZH2, leading to neuroprotection. Overall, these findings demonstrate the potential of PSELT as a therapeutic candidate for treatment of PD, targeting oxidative stress at multiple intracellular levels.
Keywords: Neurodegenerative disease; Neuron; Oxidative stress; Peptide therapy; Selenoprotein.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
There is no conflict of interest.
Figures


References
-
- Obeso J.A., Stamelou M., Goetz C.G., Poewe W., Lang A.E., Weintraub D., Burn D., Halliday G.M., Bezard E., Przedborski S., Lehericy S., Brooks D.J., Rothwell J.C., Hallett M., DeLong M.R., Marras C., Tanner C.M., Ross G.W., Langston J.W., Klein C., Bonifati V., Jankovic J., Lozano A.M., Deuschl G., Bergman H., Tolosa E., Rodriguez-Violante M., Fahn S., Postuma R.B., Berg D., Marek K., Standaert D.G., Surmeier D.J., Olanow C.W., Kordower J.H., Calabresi P., Schapira A.H.V., Stoess A.J. Past, present, and future of Parkinson's disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017;32:1264–1310. - PMC - PubMed
-
- Macleod A.D., Taylor K.S., Counsell C.E. Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 2014;29:1615–1622. - PubMed
-
- Bisaglia M., Greggio E., Beltramini M., Bubacco L. Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson's disease therapies. Faseb. J. 2013;27:2101–2110. - PubMed
-
- Hipp M.S., Kasturi P., Hartl F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019;20:421–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

