Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines
- PMID: 33486212
- PMCID: PMC7802525
- DOI: 10.1016/j.biopha.2021.111272
Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines
Abstract
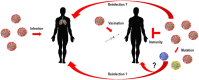
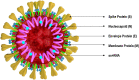
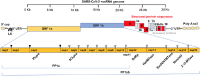
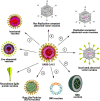
Coronavirus disease 19 (COVID-19) continues to challenge most scientists in the search of an effective way to either prevent infection or to avoid spreading of the disease. As result of global efforts some advances have been reached and we are more prepared today than we were at the beginning of the pandemic, however not enough to stop the transmission, and many questions remain unanswered. The possibility of reinfection of recovered individuals, the duration of the immunity, the impact of SARS-CoV-2 mutations in the spreading of the disease as well as the degree of protection that a potential vaccine could have are some of the issues under debate. A number of vaccines are under development using different platforms and clinical trials are ongoing in different countries, but even if they are licensed it will need time until reach a definite conclusion about their real safety and efficacy. Herein we discuss the different strategies used in the development of COVID-19 vaccines, the questions underlying the type of immune response they may elicit, the consequences that new mutations may have in the generation of sub-strains of SARS-CoV-2 and their impact and challenges for the efficacy of potential vaccines in a scenario postpandemic.
Keywords: Long-term immunity; Mutation; Reinfection; Spike protein; T-cell response.
Copyright © 2021 The Author(s). Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
I hereby declare that I have no conflict of interest.
Figures

References
-
- 2021. European Centre for Disease Prevention and Control. COVID-19 Situation Dashboard.https://www.ecdc.europa.eu/en/covid-19/situation-updates (Accessed December 7, 2020)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

