An Australian diagnostic microbiology surge response to the COVID-19 pandemic
- PMID: 33486387
- PMCID: PMC7833915
- DOI: 10.1016/j.diagmicrobio.2021.115309
An Australian diagnostic microbiology surge response to the COVID-19 pandemic
Abstract
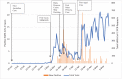
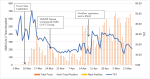
Diagnostic microbiology services form a critical component of the response to infectious disease outbreaks. Like previous respiratory virus pandemics, the COVID-19 pandemic has placed significant strains on the standing capacity of laboratories around the world. In this case study, we describe the surge response required by our laboratory to meet the fluctuating demand for SARS-CoV-2 in our regional pathology service in Western Sydney, Australia between March and May 2020. While the overall number of SARS-CoV-2 PCR positive cases was relatively low compared to other Australian local health districts, testing numbers were highly unpredictable and changed on a weekly basis as local outbreaks were detected. As with other laboratories, numerous other challenges were also faced during this period, including the requirement to introduce a new and unaccredited diagnostic PCR assay for SARS-CoV-2, local and global shortages of reagents for sampling and sample processing, and a significant institutional SARS-CoV-2 outbreak in our laboratory catchment area. A successful service delivery during this period could only be maintained by a dynamic whole-of-laboratory and organizational response including (1) operational changes to the hours of service and the expansion of diagnostic testing at our laboratory site and other sites within our organization (2) careful management of specialist staff and re-training and recruitment of additional staff (3) changes to laboratory workflows to improve SARS-CoV-2 PCR test turnaround time and to accommodate limits to precious laboratory reagents; (4) clear communication within our laboratory and the NSW Health Pathology organization; and (5) collaborative co-ordination and support by NSW Health Pathology.
Keywords: COVID-19; Laboratory management; SARS-CoV-2; Service delivery; Turnaround time.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- COVID-19 National Incident Room Surveillance Team, COVID-19 Australia Epidemology Report 23, fortnight reporting period ending 16 August 2020. Commun Dis Intell. 2020;44:1–29.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous