An Open-Label, Randomized, Controlled, Crossover Study to Assess Nicotine Pharmacokinetics and Subjective Effects of the JUUL System with Three Nicotine Concentrations Relative to Combustible Cigarettes in Adult Smokers
- PMID: 33486526
- PMCID: PMC8628869
- DOI: 10.1093/ntr/ntab001
An Open-Label, Randomized, Controlled, Crossover Study to Assess Nicotine Pharmacokinetics and Subjective Effects of the JUUL System with Three Nicotine Concentrations Relative to Combustible Cigarettes in Adult Smokers
Abstract
Introduction: This randomized, open-label, crossover clinical study evaluated nicotine pharmacokinetics (PK) and subjective effects of the JUUL System (JS; Juul Labs, Inc.) with three nicotine concentrations compared to the usual brand (UB) cigarettes in 24 adult smokers.
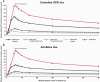
Methods: At five study visits, subjects used either the JS in 59 mg/mL, JS 18 mg/mL (two visits), and JS 9 mg/mL (all tobacco-flavored) or smoked their UB cigarette first during a controlled puffing sequence (CPS) and then ad libitum (5 min) use sessions. Blood samples were taken at specified timepoints for 60 min in each session. The modified Product Evaluation Scale assessed subjective effects 30-min post-use in the CPS session.
Results: Maximum plasma nicotine concentration (Cmax-BL), total nicotine exposure (AUC0-60-BL), and rate of plasma nicotine rise were significantly lower for all JS products compared to subjects' UB cigarette in CPS and ad libitum use sessions. In both use sessions these PK parameters were significantly higher for JS 59 mg/mL compared to 18 and 9 mg/mL. Subjective measures of cigarette craving relief and "Enough Nicotine" for JS 59 mg/mL did not differ significantly from UB cigarettes, but JS 18 and 9 mg/mL were rated significantly lower than JS 59 mg/mL and UB cigarettes.
Conclusions: Nicotine exposure and subjective relief were directly related to JS nicotine concentration: higher nicotine concentrations gave rise to significantly greater plasma nicotine levels and relief from craving. Heavier and more dependent smokers may require the greater nicotine delivery of JS 59 mg/mL to successfully transition away from cigarettes.
Implications: It has been suggested that electronic nicotine delivery systems (ENDS) and other alternative nicotine delivery products that more closely mimic the nicotine pharmacokinetics (PK) of cigarettes may facilitate smokers transitioning away from cigarettes. We examined nicotine PK and subjective effects of JUUL System (JS) ENDS with three nicotine concentrations (59, 18 and 9 mg/mL) compared to combustible cigarettes. Nicotine delivery from JS ENDS was nicotine concentration dependent, with higher nicotine concentrations giving rise to higher nicotine exposure. These findings suggest that heavier and more dependent smokers may require ENDS with nicotine concentrations greater than 20 mg/mL to successfully transition away from cigarettes.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures
References
-
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
-
- U.S. Department of Health and Human Services Medicine. Publications and Reports of the Surgeon General. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010. - PubMed
-
- Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. - PubMed
-
- Institute of Medicine National Academies of Sciences. Clearing the Smoke - Assessing the Science Base for Tobacco Harm Reduction. Washington. D.C.: The National Academies Press; 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical