Epithelial Sodium Channel and Salt-Sensitive Hypertension
- PMID: 33486988
- PMCID: PMC7878349
- DOI: 10.1161/HYPERTENSIONAHA.120.14481
Epithelial Sodium Channel and Salt-Sensitive Hypertension
Abstract
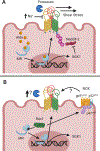
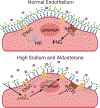
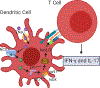
The development of high blood pressure is influenced by genetic and environmental factors, with high salt intake being a known environmental contributor. Humans display a spectrum of sodium-sensitivity, with some individuals displaying a significant blood pressure rise in response to increased sodium intake while others experience almost no change. These differences are, in part, attributable to genetic variation in pathways involved in sodium handling and excretion. ENaC (epithelial sodium channel) is one of the key transporters responsible for the reabsorption of sodium in the distal nephron. This channel has an important role in the regulation of extracellular fluid volume and consequently blood pressure. Herein, we review the role of ENaC in the development of salt-sensitive hypertension, and present mechanistic insights into the regulation of ENaC activity and how it may accelerate sodium-induced damage and dysfunction. We discuss the traditional role of ENaC in renal sodium reabsorption and review work addressing ENaC expression and function in the brain, vasculature, and immune cells, and how this has expanded the implications for its role in the initiation and progression of salt-sensitive hypertension.
Keywords: blood pressure; endothelium; extracellular fluid; nephrons; smooth muscle; sodium.
Figures
Similar articles
-
Involvement of ENaC in the development of salt-sensitive hypertension.Am J Physiol Renal Physiol. 2017 Aug 1;313(2):F135-F140. doi: 10.1152/ajprenal.00427.2016. Epub 2016 Dec 21. Am J Physiol Renal Physiol. 2017. PMID: 28003189 Free PMC article. Review.
-
Role of Rho GDP dissociation inhibitor α in control of epithelial sodium channel (ENaC)-mediated sodium reabsorption.J Biol Chem. 2014 Oct 10;289(41):28651-9. doi: 10.1074/jbc.M114.558262. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164814 Free PMC article.
-
Direct regulation of ENaC by bradykinin in the distal nephron. Implications for renal sodium handling.Curr Opin Nephrol Hypertens. 2014 Mar;23(2):122-9. doi: 10.1097/01.mnh.0000441053.81339.61. Curr Opin Nephrol Hypertens. 2014. PMID: 24378775 Free PMC article. Review.
-
Caffeine intake antagonizes salt sensitive hypertension through improvement of renal sodium handling.Sci Rep. 2016 May 12;6:25746. doi: 10.1038/srep25746. Sci Rep. 2016. PMID: 27173481 Free PMC article.
-
Control of ENaC-mediated sodium reabsorption in the distal nephron by Bradykinin.Vitam Horm. 2015;98:137-54. doi: 10.1016/bs.vh.2014.12.005. Epub 2015 Feb 14. Vitam Horm. 2015. PMID: 25817868 Review.
Cited by
-
Salt Sensitivity of Blood Pressure in Women.Hypertension. 2023 Feb;80(2):268-278. doi: 10.1161/HYPERTENSIONAHA.122.17952. Epub 2022 Aug 23. Hypertension. 2023. PMID: 35997024 Free PMC article. Review.
-
Clinical Application of Epithelial Sodium Channel (ENaC) as a Biomarker for Arterial Hypertension.Biosensors (Basel). 2022 Sep 29;12(10):806. doi: 10.3390/bios12100806. Biosensors (Basel). 2022. PMID: 36290943 Free PMC article.
-
Enterococcus faecalis: implications for host health.World J Microbiol Biotechnol. 2024 May 4;40(6):190. doi: 10.1007/s11274-024-04007-w. World J Microbiol Biotechnol. 2024. PMID: 38702495 Review.
-
Automated patch-clamp recordings for detecting activators and inhibitors of the epithelial sodium channel (ENaC).Pflugers Arch. 2025 Jun;477(6):857-872. doi: 10.1007/s00424-025-03087-3. Epub 2025 May 8. Pflugers Arch. 2025. PMID: 40335709 Free PMC article.
-
Renal lysophospholipase A1 contributes to Enterococcus faecalis-induced hypertension by enhancing sodium reabsorption.iScience. 2022 Oct 19;25(12):105403. doi: 10.1016/j.isci.2022.105403. eCollection 2022 Dec 22. iScience. 2022. PMID: 36419851 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

