Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance
- PMID: 33487630
- PMCID: PMC8080675
- DOI: 10.1038/s12276-021-00557-3
Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance
Abstract
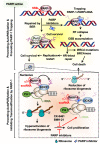
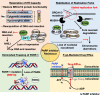
Homologous recombination (HR) repair deficiency impairs the proper maintenance of genomic stability, thus rendering cancer cells vulnerable to loss or inhibition of DNA repair proteins, such as poly(ADP-ribose) polymerase-1 (PARP-1). Inhibitors of nuclear PARPs are effective therapeutics for a number of different types of cancers. Here we review key concepts and current progress on the therapeutic use of PARP inhibitors (PARPi). PARPi selectively induce synthetic lethality in cancer cells with homologous recombination deficiencies (HRDs), the most notable being cancer cells harboring mutations in the BRCA1 and BRCA2 genes. Recent clinical evidence, however, shows that PARPi can be effective as cancer therapeutics regardless of BRCA1/2 or HRD status, suggesting that a broader population of patients might benefit from PARPi therapy. Currently, four PARPi have been approved by the Food and Drug Administration (FDA) for the treatment of advanced ovarian and breast cancer with deleterious BRCA mutations. Although PARPi have been shown to improve progression-free survival, cancer cells inevitably develop resistance, which poses a significant obstacle to the prolonged use of PARP inhibitors. For example, somatic BRCA1/2 reversion mutations are often identified in patients with BRCA1/2-mutated cancers after treatment with platinum-based therapy, causing restoration of HR capacity and thus conferring PARPi resistance. Accordingly, PARPi have been studied in combination with other targeted therapies to overcome PARPi resistance, enhance PARPi efficacy, and sensitize tumors to PARP inhibition. Moreover, multiple clinical trials are now actively underway to evaluate novel combinations of PARPi with other anticancer therapies for the treatment of PARPi-resistant cancer. In this review, we highlight the mechanisms of action of PARP inhibitors with or without BRCA1/2 defects and provide an overview of the ongoing clinical trials of PARPi. We also review the current progress on PARPi-based combination strategies and PARP inhibitor resistance.
Conflict of interest statement
W.L.K. is a founder and consultant for Ribon Therapeutics, Inc. He is also a coholder of U.S. Patent 9,599,606 covering a set of ADP-ribose detection reagents, which have been licensed to and are sold by EMD Millipore.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

