Comparison of Dry-Powder Inhaler and Pressurized Metered-Dose Inhaler Formulations of Extrafine Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium in Patients with COPD: The TRI-D Randomized Controlled Trial
- PMID: 33488071
- PMCID: PMC7814657
- DOI: 10.2147/COPD.S291030
Comparison of Dry-Powder Inhaler and Pressurized Metered-Dose Inhaler Formulations of Extrafine Beclomethasone Dipropionate/Formoterol Fumarate/Glycopyrronium in Patients with COPD: The TRI-D Randomized Controlled Trial
Abstract
Background: Three 52-week studies in COPD have assessed the efficacy and safety of single-inhaler extrafine formulation triple therapy combining beclomethasone dipropionate (BDP), formoterol fumarate (FF) and glycopyrronium (G) delivered via pressurized metered-dose inhaler (pMDI). BDP/FF/G is now being developed for delivery via multi-dose dry-powder inhaler (DPI; NEXThaler). This study aimed to demonstrate non-inferiority of BDP/FF/G DPI vs pMDI for lung function.
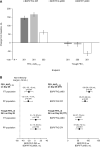
Methods: Multicenter, randomized, double-blind, double-dummy, active-controlled, three-way cross-over study in patients with COPD and post-bronchodilator forced expiratory volume in 1 second (FEV1) 30-80% predicted. Patients received BDP/FF/G 100/6/10µg via DPI and pMDI, and BDP/FF 100/6µg via pMDI, all two inhalations twice daily for four weeks, with treatments separated by two-week washout. The two co-primary objectives were to demonstrate non-inferiority between the two BDP/FF/G formulations for FEV1 area under the curve between 0 and 12 hours post-dose (AUC0-12h) normalized by time and trough FEV1 at 24 hours, both on Day 28. EudraCT 2017-004405-41.
Results: Of 449 patients screened, 366 were randomized, with 342 (93.4%) completing all three treatment periods. The primary objectives were met, with changes from baseline in FEV1 AUC0-12h and trough FEV1 on Day 28 similar for the two BDP/FF/G formulations, and the confidence intervals for the difference lying entirely within the pre-specified non-inferiority criterion (-50mL): -20 (-35, -6) mL and 3 (-15, 20) mL for AUC0-12h and trough FEV1, respectively. BDP/FF/G pMDI and DPI were statistically superior to BDP/FF for these endpoints (p<0.001). A similar proportion of patients experienced adverse events with each treatment (15.5%, 18.7% and 15.4% with BDP/FF/G DPI and pMDI, and BDP/FF, respectively); the majority were mild or moderate, with few related to treatment.
Conclusion: Extrafine BDP/FF/G DPI and pMDI demonstrated similar efficacy and safety in patients with COPD, supporting the DPI formulation as a valid alternative.
Keywords: adrenergic beta-2 receptor agonists; chronic obstructive pulmonary disease; muscarinic antagonists; respiratory function tests; steroids.
© 2021 Beeh et al.
Conflict of interest statement
KMB is a full time employee of insaf Respiratory Research Institute. He has received personal or institutional compensations for services on advisory boards or consulting for AstraZeneca, Berlin Chemie, Boehringer, Chiesi, Elpen, GSK, Mundipharma, Novartis, Pohl Boskamp, Sanofi, sterna and Zentiva, and compensations for speaker activities in scientific meetings supported by AstraZeneca, Berlin Chemie, Boehringer, ERT, GSK, Novartis, Pfizer, Pohl Boskamp, Sanofi and Teva, all outside the submitted work. The institution has received compensations for design and performance of clinical trials from AstraZeneca, Boehringer, Chiesi, GSK, Novartis, Parexel, Pearl Therapeutics, sterna, and Zentiva. PK reports personal fees from Chiesi, Novartis, AstraZeneca, Boehringer Ingelheim, Berlin Chemie Menarini, Adamed, Polpharma and Lekam, all outside the submitted work and reports lectures for Novartis, GSK, Teva, AstraZeneca, Polpharma, and Lekam, outside the submitted work. MC received grants and honoraria for lectures from Chiesi Farmaceutici SpA, outside the submitted work. IV, AG and GG are employees of Chiesi Farmaceutici SpA, the sponsor of the study. The authors report no other potential conflicts of interest for this work.
Figures




References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Published 2021. Available from: https://goldcopd.org/2021-gold-reports/. Accessed November25, 2020.
-
- Braido F, Scichilone N, Lavorini F, et al. Manifesto on small airway involvement and management in asthma and chronic obstructive pulmonary disease: an Interasma (Global Asthma Association - GAA) and World Allergy Organization (WAO) document. World Allergy Organ J. 2016;9(1):1–6. doi: 10.1186/s40413-016-0123-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

