2-[(3-Aminoalkyl-(alkaryl-,aryl-))-1H-1,2,4-triazol-5-yl]anilines: synthesis and anticonvulsant activity
- PMID: 33488191
- PMCID: PMC7671191
- DOI: 10.3906/kim-2002-24
2-[(3-Aminoalkyl-(alkaryl-,aryl-))-1H-1,2,4-triazol-5-yl]anilines: synthesis and anticonvulsant activity
Abstract
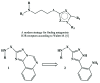
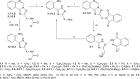
The presented work is devoted to the development of synthesis methods for novel 2-[(3-aminoalkyl-(alkaryl-, aryl-))-1H-1,2,4-triazolo]anilines. Abovementioned compounds were obtained via hydrazinolysis (Ing-Manske procedure) and acid hydrolysis of corresponding N -acylated{([1,2,4]triazolo[1,5-c]quinazolin-2-yl)alkyl-(alkaryl-, aryl-)}amines. The regioselectivity of hydrazinolysis and hydrolysis were established. The features of spectral characteristics werestudied and discussed. Characteristic patterns of protons signals splitting in 1H NMR of the synthesized compounds were established. The effect of the synthesized compounds on the pentylenetetrazol seizures was studied. It was found that according to some indicators, anticonvulsant activity of 2-[(3-aminoalkyl-(alkaryl-, aryl-))-1H-1,2,4-triazolo]anilines superior or comparable with effect of the reference drug "Lamotrigine". It is a valid argument for their further structural modification, in-depth study of activity mechanisms and further study of anticonvulsant activity on other experimental seizures models.
Keywords: 2-[(3-aminoalkyl-(alkaryl-,aryl-))-1H-1,2,4-triazolo]anilines; N -acylated{([1,2,4]triazolo[1,5-c]quinazolin-2-yl)alkyl-(alkaryl-, aryl-)}amines; acidic hydrolysis; anticonvulsant activity; hydrazinolysis.
Copyright © 2020 The Author(s).
Conflict of interest statement
CONFLICT OF INTEREST: none declared.
Figures
References
-
- Histamine Receptors: Preclinical and Clinical Aspects. The Receptors 2016.
LinkOut - more resources
Full Text Sources