Preparation, structure elucidation, and antioxidant activity of new bis(thiosemicarbazone) derivatives
- PMID: 33488214
- PMCID: PMC7751920
- DOI: 10.3906/kim-2002-76
Preparation, structure elucidation, and antioxidant activity of new bis(thiosemicarbazone) derivatives
Abstract
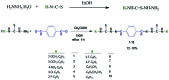
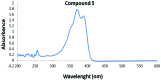
Schiff-base-bearing new bis(thiosemicarbazone) derivatives were prepared from terephthalaldehyde and various thiosemicarbazides. FT-IR, 1H NMR, 13C NMR, and UV-Vis spectroscopic methods and elemental analysis were used to elucidate the identification of the synthesized molecules. The in vitro antioxidant activity of the synthesized compounds was analysed with the 1,1-diphenyl-2-picryl hydrazyl free-radical-trapping process. The synthesized compounds exhibited lower antioxidant activity than the standard ascorbic acid. IC50 values of the synthesized molecules measured from 3.81 ± 0.01 to 29.05 ± 0.11 μM. Among the synthesized compounds, compound 3 had the best antioxidant activity. Moreover, this study explained the structure-activity relationship of the synthesized molecules with different substituents in radical trapping reactions.
Keywords: DPPH; Schiff bases; Thiosemicarbazides; spectroscopic methods; structure–activity relationship.
Copyright © 2020 The Author(s).
Conflict of interest statement
CONFLICT OF INTEREST: The author declares that he has no conflict of interest.
Figures



Similar articles
-
Synthesis, spectroscopic studies, and antioxidant activities of novel thio/carbohydrazones and bis-isatin derivatives from terephthalaldehyde.Turk J Chem. 2020 Feb 11;44(1):237-248. doi: 10.3906/kim-1910-13. eCollection 2020. Turk J Chem. 2020. PMID: 33488154 Free PMC article.
-
Synthesis, characterization, spectroscopic studies and biological evaluation of Schiff bases derived from 1-hydroxy-2-acetonapthanone.Heliyon. 2019 Nov 14;5(11):e02774. doi: 10.1016/j.heliyon.2019.e02774. eCollection 2019 Nov. Heliyon. 2019. PMID: 31763472 Free PMC article.
-
Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives.Carbohydr Polym. 2018 Feb 1;181:812-817. doi: 10.1016/j.carbpol.2017.11.074. Epub 2017 Nov 24. Carbohydr Polym. 2018. PMID: 29254040
-
Schiff's bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants.Bioorg Med Chem Lett. 2015 Mar 1;25(5):1072-7. doi: 10.1016/j.bmcl.2015.01.010. Epub 2015 Jan 13. Bioorg Med Chem Lett. 2015. PMID: 25638040 Review.
-
Synthesis, characterization, computational, antioxidant and fluorescence properties of novel 1,3,5-trimesic hydrazones derivatives.Heliyon. 2021 Sep 25;7(9):e08074. doi: 10.1016/j.heliyon.2021.e08074. eCollection 2021 Sep. Heliyon. 2021. PMID: 34622075 Free PMC article. Review.
Cited by
-
Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates.Int J Mol Sci. 2022 Jul 12;23(14):7688. doi: 10.3390/ijms23147688. Int J Mol Sci. 2022. PMID: 35887038 Free PMC article.
-
Kinetic Insights into the Antioxidant Effect of Isatin-Thiosemicarbazone in Biodiesel Blends.Antioxidants (Basel). 2024 Jul 8;13(7):819. doi: 10.3390/antiox13070819. Antioxidants (Basel). 2024. PMID: 39061888 Free PMC article.
References
-
- Aly MM Mohamed YA El-Bayouki KA Basyouni WM Abbas SY Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities-Part-1. European Journal of Medicinal Chemistry . 2010;45:3365–3373. - PubMed
-
- Sriram D Yogeeswari P Thirumurugan R Pavana RK Discovery of new antitubercular oxazolyl thiosemicarbazones. Journal of Medicinal Chemistry . 2006;49:3448–3450. - PubMed
-
- Pervez H Saira N Iqbal MS Yaqub M Khan KM Synthesis and biological evaluation of some $N^{4}$-aryl-substituted-5-fluoroisatin-3-thiosemicarbazones. Medicinal Chemistry Research . 2013;22:5878–5889.
-
- Stanojkovic TP Kovala-Demertzi D Primikyri A Garcia-Santos I Castineiras A Zinc (II) complexes of 2-acetyl pyridine 1-(4-fluorophenyl)-piperazinyl thiosemicarbazone: synthesis, spectroscopic study and crystal structures-potential anticancer drugs. Journal of Inorganic Biochemistry . 2010;104:467–476. - PubMed
-
- Güveli Ş Turan K Ülküseven B Nickel -PPh$_{3}$ complexes with ONS and ONN chelating thiosemicarbazones: synthesis and inhibition potential on influenza A viruses. Turkish Journal of Chemistry . 2018;42:371–384.
LinkOut - more resources
Full Text Sources
Miscellaneous
