Extracellular Vesicles Derived From Talaromyces marneffei Yeasts Mediate Inflammatory Response in Macrophage Cells by Bioactive Protein Components
- PMID: 33488545
- PMCID: PMC7819977
- DOI: 10.3389/fmicb.2020.603183
Extracellular Vesicles Derived From Talaromyces marneffei Yeasts Mediate Inflammatory Response in Macrophage Cells by Bioactive Protein Components
Abstract
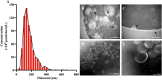
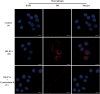
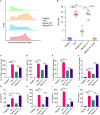
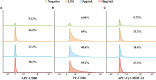
Extracellular vesicles (EVs) loaded with proteins, nucleic acids, membrane lipids, and other virulence factors could participate in pathogenic processes in some fungi such as Cryptococcus neoformans and Candida albicans. However, the specific characteristics of EVs derived from Talaromyces marneffei (TM) still have not been figured out yet. In the present study, it has been observed that TM-derived EVs were a heterogeneous group of nanosized membrane vesicles (30-300 nm) under nanoparticle tracking analysis and transmission electron microscopy. The DiI-labeled EVs could be taken up by RAW 264.7 macrophage cells. Incubation of EVs with macrophages would result in increased expression levels of reactive oxygen species, nitric oxide, and some inflammatory factors including interleukin-1β, interleukin-6, interleukin-10, and tumor necrosis factor. Furthermore, the expression of co-stimulatory molecules (CD80, CD86, and MHC-II) was also increased in macrophages stimulated with EVs. The level of inflammatory factors secreted by macrophages showed a significant decrease when EVs were hydrolyzed by protease, while that of DNA and RNA hydrolase treatment remained unchanged. Subsequently, some virulence factors in EVs including heat shock protein, mannoprotein 1, and peroxidase were determined by liquid chromatography-tandem mass spectrometry. Taken together, our results indicated that the TM-derived EVs could mediate inflammatory response and its protein would play a key role in regulating the function of RAW 264.7 macrophage cells.
Keywords: Talaromyces marneffei; extracellular vesicles; inflammatory response; macrophage cells; proteomics.
Copyright © 2021 Yang, Wang, Jiang, Lin, Ou, Ullah, Hua, Chen, Lin, Hu, Zheng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Extracellular vesicles derived from Talaromyces marneffei contain immunogenic compounds and modulate THP-1 macrophage responses.Front Immunol. 2023 Jun 29;14:1192326. doi: 10.3389/fimmu.2023.1192326. eCollection 2023. Front Immunol. 2023. PMID: 37457708 Free PMC article.
-
A Novel Protocol for the Isolation of Fungal Extracellular Vesicles Reveals the Participation of a Putative Scramblase in Polysaccharide Export and Capsule Construction in Cryptococcus gattii.mSphere. 2019 Mar 20;4(2):e00080-19. doi: 10.1128/mSphere.00080-19. mSphere. 2019. PMID: 30894430 Free PMC article.
-
Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival.Front Immunol. 2018 Feb 19;9:272. doi: 10.3389/fimmu.2018.00272. eCollection 2018. Front Immunol. 2018. PMID: 29520273 Free PMC article.
-
The Emerging Roles of Extracellular Vesicles in Osteosarcoma.Front Oncol. 2019 Dec 3;9:1342. doi: 10.3389/fonc.2019.01342. eCollection 2019. Front Oncol. 2019. PMID: 31850225 Free PMC article. Review.
-
Fungal Extracellular Vesicles with a Focus on Proteomic Analysis.Proteomics. 2019 Apr;19(8):e1800232. doi: 10.1002/pmic.201800232. Epub 2019 Apr 2. Proteomics. 2019. PMID: 30883019 Review.
Cited by
-
The emerging role of extracellular vesicles in fungi: a double-edged sword.Front Microbiol. 2023 Jul 18;14:1216895. doi: 10.3389/fmicb.2023.1216895. eCollection 2023. Front Microbiol. 2023. PMID: 37533824 Free PMC article. Review.
-
Hidden allies: how extracellular vesicles drive biofilm formation, stress adaptation, and host-immune interactions in human fungal pathogens.mBio. 2024 Dec 11;15(12):e0304523. doi: 10.1128/mbio.03045-23. Epub 2024 Nov 18. mBio. 2024. PMID: 39555918 Free PMC article. Review.
-
Clinical and chest CT manifestations of Talaromycosis marneffei: a retrospective study comparing anti-IFN-γ autoantibodies-positive and HIV-positive cases.BMC Infect Dis. 2024 Nov 28;24(1):1357. doi: 10.1186/s12879-024-10255-w. BMC Infect Dis. 2024. PMID: 39609734 Free PMC article.
-
Talaromyces marneffei central nervous system infection unveiled by the novel Mp1p antigen detection assay in AIDS patient.BMC Infect Dis. 2024 Dec 23;24(1):1456. doi: 10.1186/s12879-024-10336-w. BMC Infect Dis. 2024. PMID: 39710631 Free PMC article.
-
Extracellular vesicles of Candida albicans regulate its own growth through the L-arginine/nitric oxide pathway.Appl Microbiol Biotechnol. 2023 Jan;107(1):355-367. doi: 10.1007/s00253-022-12300-7. Epub 2022 Nov 28. Appl Microbiol Biotechnol. 2023. PMID: 36441207 Free PMC article.
References
-
- Albuquerque P. C., Nakayasu E. S., Rodrigues M. L., Frases S., Casadevall A., Zancope-Oliveira R. M., et al. (2008). Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 10 1695–1710. 10.1111/j.1462-5822.2008.01160.x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

