Immunodiagnosis and Immunotherapeutics Based on Human Papillomavirus for HPV-Induced Cancers
- PMID: 33488587
- PMCID: PMC7820759
- DOI: 10.3389/fimmu.2020.586796
Immunodiagnosis and Immunotherapeutics Based on Human Papillomavirus for HPV-Induced Cancers
Abstract
Infection with human papillomavirus (HPV) is one of the main causes of malignant neoplasms, especially cervical, anogenital, and oropharyngeal cancers. Although we have developed preventive vaccines that can protect from HPV infection, there are still many new cases of HPV-related cancers worldwide. Early diagnosis and therapy are therefore important for the treatment of these diseases. As HPVs are the major contributors to these cancers, it is reasonable to develop reagents, kits, or devices to detect and eliminate HPVs for early diagnosis and therapeutics. Immunological methods are precise strategies that are promising for the accurate detection and blockade of HPVs. During the last decades, the mechanism of how HPVs induce neoplasms has been extensively elucidated, and several oncogenic HPV early proteins, including E5, E6, and E7, have been shown to be positively related to the oncogenesis and malignancy of HPV-induced cancers. These oncoproteins are promising biomarkers for diagnosis and as targets for the therapeutics of HPV-related cancers. Importantly, many specific monoclonal antibodies (mAbs), or newly designed antibody mimics, as well as new immunological kits, devices, and reagents have been developed for both the immunodiagnosis and immunotherapeutics of HPV-induced cancers. In the current review, we summarize the research progress in the immunodiagnosis and immunotherapeutics based on HPV for HPV-induced cancers. In particular, we depict the most promising serological methods for the detection of HPV infection and several therapeutical immunotherapeutics based on HPV, using immunological tools, including native mAbs, radio-labelled mAbs, affitoxins (affibody-linked toxins), intracellular single-chain antibodies (scFvs), nanobodies, therapeutical vaccines, and T-cell-based therapies. Our review aims to provide new clues for researchers to develop novel strategies and methods for the diagnosis and treatment of HPV-induced tumors.
Keywords: cervical cancer; human papillomavirus; immunodiagnosis; immunotherapeutics; monoclonal antibody.
Copyright © 2021 Dong, Hu, Du, Tan, Li, Du, Bai, Ma and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



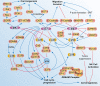

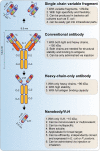
References
-
- Rajendra S, Xuan W, Merrett N, Sharma P, Sharma P, Pavey D, et al. Survival Rates for Patients With Barrett High-grade Dysplasia and Esophageal Adenocarcinoma With or Without Human Papillomavirus Infection. JAMA Network Open (2018) 1:e181054–e181054. 10.1001/jamanetworkopen.2018.1054 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

