Transcriptomic and Network Analysis of Minor Salivary Glands of Patients With Primary Sjögren's Syndrome
- PMID: 33488608
- PMCID: PMC7821166
- DOI: 10.3389/fimmu.2020.606268
Transcriptomic and Network Analysis of Minor Salivary Glands of Patients With Primary Sjögren's Syndrome
Abstract
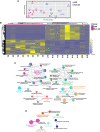
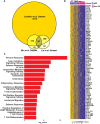
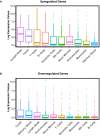
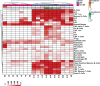
Primary Sjögren's syndrome (pSS) is a systemic autoimmune disease characterized primarily by immune-mediated destruction of exocrine tissues, such as those of the salivary and lacrimal glands, resulting in the loss of saliva and tear production, respectively. This disease predominantly affects middle-aged women, often in an insidious manner with the accumulation of subtle changes in glandular function occurring over many years. Patients commonly suffer from pSS symptoms for years before receiving a diagnosis. Currently, there is no effective cure for pSS and treatment options and targeted therapy approaches are limited due to a lack of our overall understanding of the disease etiology and its underlying pathology. To better elucidate the underlying molecular nature of this disease, we have performed RNA-sequencing to generate a comprehensive global gene expression profile of minor salivary glands from an ethnically diverse cohort of patients with pSS. Gene expression analysis has identified a number of pathways and networks that are relevant in pSS pathogenesis. Moreover, our detailed integrative analysis has revealed a primary Sjögren's syndrome molecular signature that may represent important players acting as potential drivers of this disease. Finally, we have established that the global transcriptomic changes in pSS are likely to be attributed not only to various immune cell types within the salivary gland but also epithelial cells which are likely playing a contributing role. Overall, our comprehensive studies provide a database-enriched framework and resource for the identification and examination of key pathways, mediators, and new biomarkers important in the pathogenesis of this disease with the long-term goals of facilitating earlier diagnosis of pSS and to mitigate or abrogate the progression of this debilitating disease.
Keywords: RNA-sequencing; Sjögren’s syndrome; bioinformatics; gene expression; salivary gland.
Copyright © 2021 Oyelakin, Horeth, Song, Min, Che, Marzullo, Lessard, Rasmussen, Radfar, Scofield, Lewis, Stone, Grundahl, De Rossi, Kurago, Farris, Sivils, Sinha, Kramer and Romano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
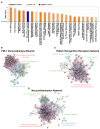
References
-
- Flores-Chavez A, Kostov B, Solans R, Fraile G, Maure B, Feijoo-Masso C, et al. Severe, life-threatening phenotype of primary Sjogren’s syndrome: clinical characterisation and outcomes in 1580 patients (GEAS-SS Registry). Clin Exp Rheumatol (2018) 36 Suppl 112(3):121–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

