Antigen-Presenting Cells in Food Tolerance and Allergy
- PMID: 33488627
- PMCID: PMC7821622
- DOI: 10.3389/fimmu.2020.616020
Antigen-Presenting Cells in Food Tolerance and Allergy
Abstract
Food allergy now affects 6%-8% of children in the Western world; despite this, we understand little about why certain people become sensitized to food allergens. The dominant form of food allergy is mediated by food-specific immunoglobulin E (IgE) antibodies, which can cause a variety of symptoms, including life-threatening anaphylaxis. A central step in this immune response to food antigens that differentiates tolerance from allergy is the initial priming of T cells by antigen-presenting cells (APCs), primarily different types of dendritic cells (DCs). DCs, along with monocyte and macrophage populations, dictate oral tolerance versus allergy by shaping the T cell and subsequent B cell antibody response. A growing body of literature has shed light on the conditions under which antigen presentation occurs and how different types of T cell responses are induced by different APCs. We will review APC subsets in the gut and discuss mechanisms of APC-induced oral tolerance versus allergy to food identified using mouse models and patient samples.
Keywords: Peyer’s patches; dendritic cells; food allergy; gut; macrophages; mesenteric lymph node; monocytes; oral tolerance.
Copyright © 2021 Liu, Yin, Swaminathan and Eisenbarth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
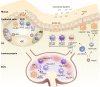
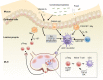
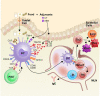
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

