Allogeneic CAR Cell Therapy-More Than a Pipe Dream
- PMID: 33488631
- PMCID: PMC7821739
- DOI: 10.3389/fimmu.2020.618427
Allogeneic CAR Cell Therapy-More Than a Pipe Dream
Abstract
Adoptive cellular immunotherapy using immune cells expressing chimeric antigen receptors (CARs) has shown promise, particularly for the treatment of hematological malignancies. To date, the majority of clinically evaluated CAR cell products have been derived from autologous immune cells. While this strategy can be effective it also imposes several constraints regarding logistics. This includes i) availability of center to perform leukapheresis, ii) necessity for shipment to and from processing centers, and iii) time requirements for product manufacture and clinical release testing. In addition, previous cytotoxic therapies can negatively impact the effector function of autologous immune cells, which may then affect efficacy and/or durability of resultant CAR products. The use of allogeneic CAR cell products generated using cells from healthy donors has the potential to overcome many of these limitations, including through generation of "off the shelf" products. However, allogeneic CAR cell products come with their own challenges, including potential to induce graft-versus-host-disease, as well as risk of immune-mediated rejection by the host. Here we will review promises and challenges of allogeneic CAR immunotherapies, including those being investigated in preclinical models and/or early phase clinical studies.
Keywords: CAR; allogeneic; cancer; cell therapy; immunotherapy.
Copyright © 2021 Caldwell, Gottschalk and Talleur.
Conflict of interest statement
SG has patent applications in the field of immunotherapy, is a DSMB member of Immatics, and on the scientific advisory board of Tidal. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


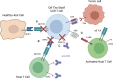
References
-
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA (1993) 90(2):720–4. 10.1073/pnas.90.2.720 - DOI - PMC - PubMed
-
- Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc (1989) 21(1 Pt 1):127–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

