Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues
- PMID: 33488736
- PMCID: PMC7787857
- DOI: 10.1155/2020/8810813
Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues
Abstract
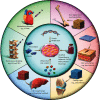
Adipose-derived stem cells (ADSCs) can maintain self-renewal and enhanced multidifferentiation potential through the release of a variety of paracrine factors and extracellular vesicles, allowing them to repair damaged organs and tissues. Consequently, considerable attention has increasingly been paid to their application in tissue engineering and organ regeneration. Here, we provide a comprehensive overview of the current status of ADSC preparation, including harvesting, isolation, and identification. The advances in preclinical and clinical evidence-based ADSC therapy for bone, cartilage, myocardium, liver, and nervous system regeneration as well as skin wound healing are also summarized. Notably, the perspectives, potential challenges, and future directions for ADSC-related researches are discussed. We hope that this review can provide comprehensive and standardized guidelines for the safe and effective application of ADSCs to achieve predictable and desired therapeutic effects.
Copyright © 2020 Jiaxin Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

